For earlier entries in my paleo research tips series, see the links below:
Paleontological Research Tips I: field notes for amateurs and professionals alike
Paleontological Research Tips II: field notes, continued
Paleontological Research Tips III: a complete idiot's guide to taking decent specimen photographs
Paleontological Research Tips IV: the art and science of maintaining a research notebook
Paleontological Research tips V: manuscript writing, research productivity, peer review, and minefield navigation
The goal of photogrammetry in paleontology is to produce a complete 3D model of a fossil to share digitally, and be of sufficient accuracy so that measurements can be recorded off of the digital model remotely. I will break this tutorial up into several sections: 1) basics for doing a single sided fossil; 2) combining two or more photo sets to produce a model isolated from whatever it was sitting on; and 3) using a turntable to produce models of fossils as small as 1 cm in diameter with one or more sets of photos; 4) scaling your STL file.
This guide to photogrammetry is intended for folks who are interested and perhaps familiar with using cameras and have a bit of gear. I've tried to keep this relatively free of jargon, which is great for beginners, but in reality is enforced by the fact that I don't really know much about the science/engineering behind photogrammetry, and quite frankly, I don't really care so long as it works. I did very little reading ahead of time, because at an earlier stage I 1) felt like nothing I was recommended to do worked very well and 2) being a scientist, I thought, why not try figuring things out through trial and error? As a result, a number of my methods converged strongly on those developed elsewhere, such as my colleague Heinrich Mallison - I recall during a talk of his at the 2018 SVP meeting in Albuquerque trying to stifle my own laughter, because he described many of the same struggles I had dealt with and overcome in more or less the same way. And, also, some solutions of his that I had not thought of, and thought to myself "DUH, of course that would work, why didn't I think of that?". So, some of this was probably unnecessary reinvention of the wheel, but by going through and failing at so many different methods and eventually succeeding, it gave me a nice feel for the limitations and possibilities of different set ups, strategies, and the technology/software itself that I would NOT have gotten if I had done what you're doing right now (reading how somebody else did it). Also, until that 2018 talk, for god knows what reason I was totally unaware of Heinrich's work.
Most importantly, you will NOT need to spend a single second in photoshop! One way to photogrammetize objects is to delete the background from every photo, forcing the computer to match points on the fossil itself. I have done this! And it SUCKS. It is extremely time intensive and mind numbing; if you don’t want to mess around with running scripts/batch edits – there is an easier way. It requires a few minor tweaks to the setup, but makes photogrammetry run pretty smoothly. I’ve spent a LOT of time experimenting, failing, and succeeding with photogrammetry over the past five years and these instructions and tips are a distillation of what I have learned.
Before progressing, I want to take the opportunity to thank Luke Easterbrook, former scientific illustrator at the University of Otago Geology Department, who taught me photogrammetry; and second, Dr. Norm Levine in our department at CofC, who has helped me with innumerable technical issues as well as software. I wrote this post for some of my preparator/student colleagues at University of Otago, since I did not pass on what I had learned then, given the very steep learning curve and unfortunate methods I was using at the time (see above, photoshop).
Want to see many of the 3D models made by my students and I over the past few years? We've uploaded many of the models that are not research-sensitive (e.g. currently under study and therefore, under wraps) and they can be viewed (and some downloaded) here.
Disclaimer: This post *only* refers to use of AGI photoscan. I have no experience using other programs, and am not aware of the most likely glitches you would encounter with them and therefore these strategies to trick the computer may or may not work (I am willing to bet good money that they will). As a result, I will be completely unaware to help out with technical support for anything like 123D catch, autodesk, or others.
1) Single sided fossils and basics
This tutorial assumes you are using Agisoft Photoscan – the only software I really know how to use. Aside from issues with the GUI of the software itself, everything else *should* apply to other software.
Camera settings. Many fossils that are exposed in relief can be easily photogrammetized with a handheld DSLR camera. If it’s a large fossil, a zoom lens is usually best; you’ll want to make sure that the aperture is set so that most of the specimen is in focus (wide depth of field) – so a higher f number (f/16 or higher is good). Since you’re shooting handheld, make sure shutterspeed is sufficient to prevent camera shake (1/60th of a second is about as slow as you can realistically get; anything faster than 1/100th is ideal). This may mean either increasing ISO or grabbing more lights to illuminate the fossil (or both) to achieve optimum balance of all variables. It’s important to note that AGI photoscan can work even with partially blurry photos, though some surface detail is probably lost. You do not need to use RAW photo files, so make sure to set your image quality to jpg only, and on the largest image setting. Photoscan doesn’t use RAW photos, and adjusting the levels in every photograph in a RAW photo editing program would take forever and certainly qualify you to be admitted to a mental institution.
You could *probably* do this with a smartphone, but it’s probably faster to use a real camera (DSLR) with an SD card so you can drag and drop the photos more quickly (also, no internet connection needed). There’s also a huge difference in camera functionality. There are two basic camera/user types: “dumb” camera with high functionality and smart user (e.g. DSLR) or “smart” camera with “dumb” user (smartphone, point and shoot, auto function on a DSLR). This goes a LOT faster if you know how to properly use a DSLR and therefore fall within the “smart” category and can use a camera with a steep learning curve. Since this involves taking LOTS of photos, I like to use the A/V mode (aperture priority) which lets you set the aperture diameter (f-stop #) and the camera automatically then sets shutterspeed for you. This is important, as an irregularly shaped but dark object will result in either more (small side facing camera) or less light (e.g. large side facing camera) entering the camera as it moves around the object, thereby changing exposure. Under manual settings, you have to set ISO, shutterspeed, and aperture yourself, and I’m so scatterbrained even during regular technical photography I forget to re-set shutterspeed so I sort of perpetually use A/V mode anyway. Take a look at my paleontological photography guide for more information: http://coastalpaleo.blogspot.com/2016/02/paleontological-research-tips-part-3.html
Bullseye and Crosshairs shooting strategies.
Shooting strategy. When shooting, it is best to be systematic to ensure even coverage. I tend to take handheld pictures in two ways: Bullseye and Crosshairs.
Bullseye method: a series of three orbits around the fossil, with the first set at a low angle with 50-60 photos, a second set looking down at a 45 degree angle and with about 20-30 photos, and a third set that is close to 80 degrees and with about 10-15 photos. For a single sided fossil in relief, 150 photos is sufficient; past that, the processing time increases exponentially and is quite literally a case of diminishing returns. For example: a bit of extra detail is possible at 200 or 250 photos, but takes perhaps 20-30% longer. 350 photos requires perhaps double the processing time, with relatively little improvement in detail over 250. 400 is right out.
Crosshair method: using the first low angle circle, and then taking two arcs of shots over the specimen at a 90 degree angle to each other.
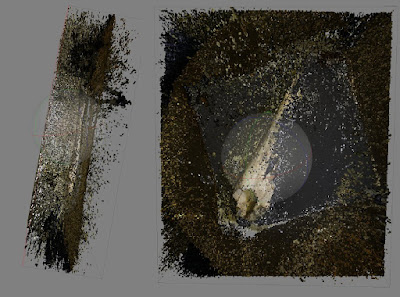
Aligned cameras (blue squares) and 3D model of a partial Capybara skull, showing two aligned photo sets using the "crosshairs" method (look for the X). See below for using multiple photo sets.
When finished, it may be wise to take some supplementary shots of any crevices or pockets; some additional illumination may be needed, and the change in lighting probably won’t affect the outcome.
Why be systematic? This is mostly to ensure that coverage is even, and that no parts are underrepresented (perhaps OK in some cases), and perhaps most critically, to speed things up. If you’re just willy nilly taking photos it is easy to think “maybe I didn’t get that spot…” and mess around for a few minutes. If it’s systematic and a routine, then you can do it faster.
Lighting/background setup. When shooting a one-sided fossil, it should be well-illuminated, but the background is not very important. Anything that is poorly lit will tend to have a bubbly texture that unfortunately is best described as resembling smallpox lesions. If you see this, the only way to go about fixing this is to re-shoot with either 1) increased lighting or 2) higher ISO or otherwise messing around with the settings on your camera (note: unusually smooth, often plaster/painted surfaces on reconstructed parts, will often also get this anomalous bumpy texture). If time is not an issue, and you have a big fossil in a dank, dark basement, you can use a tripod and corded/remote shutter button thereby eliminating camera shake. If you have a fossil that is not flat, you can also set it up on a pedestal if you want to get the sides (and not just the top) of it in the 3D model. Note that the pedestal will become part of the point cloud and therefore part of the model at the end, which you can delete – because in this case, we’re only concerned with a single sided fossil.
Things to avoid. with a zoom lens and a large fossil, it’s probably inevitable to get part of a shoe or a coworker in the background in a couple photos; that’s probably fine. Because the program matches points on everything, however, if something in at least 1/3 of the photos is moved or jostled halfway through, that can cause a number of issues, especially for part 2 and 3 of this tutorial. If you bump into a light stand, the program will only be able to use that feature to match points on for half the photos, and it may cause erroneous point matching. If a light is battery powered and goes off halfway through, that probably won’t be a problem; photoscan seems to hold up to light changes pretty well. If the fossil moves halfway through, delete all your photos, and start over.
Using the program. Make sure to copy all the photos onto a computer, and one folder per element. For parts of a multielement skeleton, I keep them in different subfolders with the specimen number, taxon name, and element name to keep everything straight.
The sparse point cloud will look like this: most of the points will either be the fossil itself, and very likely the background/tabletop and whatever it was sitting on.
Step 1: Open up Photoscan, click Workflow > add photos, and navigate to the correct folder. They will load quickly. Once loaded, go Workflow > align photos. This is the first round of photo matching, and it attempts to reconstruct the orientation and distance of camera relative to the object when you took the photos. With enough coverage, for a single sided object, there are rarely failures at this stage. Nevertheless, take a look at the ‘cameras’ – the blue squares showing the distribution of the camera – and if any look too far or too close, double check them. You can find badly aligned photos and click ‘disable camera’ using the photo pane at the bottom of the screen. In the middle of the blue squares should be the sparse point cloud – these are points used to align the cameras in three dimensions, no doubt using triangulation, but I don’t really know. Aligning photos generally takes 30 seconds per photo in the set, increasing steadily once you surpass 300 photos. Make sure to save before progressing to the next step, because you will in all likelihood need to wait several hours or overnight before you can save again.
A particularly messy looking dense point cloud - this took perhaps 30-40 minutes to clean up using the lasso tool.
Step 2: If ready, go Workflow > build Dense Point Cloud. This now produces a much more dense cloud of points now that the distribution of the camera is established. This is the longest step in the process, perhaps one minute per photo, though it is far more exponential after 200 photos. I frequently let this run overnight, as it can take many hours, and this is the step that requires the most computing power. Once completed, you will see your fossil! Zoom in, and try to delete anything that doesn’t belong, because if you leave it, it will become part of the model. Once completed, save your file.
Step 3: Once the dense point cloud is edited, go Workflow > Build Mesh. This will take every point and add a triangular surface between each point. The more erroneous points, the rougher and more inaccurate this surface will be. This is a pretty quick step, perhaps taking on average 10 seconds per photo. Save your file. At this point, you can now save it as an STL file – the standard 3D file you can share with colleagues or upload to a website like Sketchfab. Go to File > Export as and click ‘.stl’ as the file type.
Step 4: This last step is optional, but involves producing a color “texture” for the 3D model. In 3D computer work, for god knows what reason, texture doesn’t refer to the surface texture we usually think of as anatomists, but rather the color values applied to the mesh. For this step, the computer goes back to the photos and maps small parts of the photos onto the mesh. Go to Workflow > Build Texture; this is the fastest step and takes perhaps 5 seconds per photo. This image is not saved in a normal STL file. If you want to save this in a format usable by *most* people, save it as a 3D pdf. Go to File > Export as and click ‘3d pdf’ as the file type.
Different stages of building a 3D model of a fossil dolphin braincase in AGI Photoscan, which for some reason is working backwards. Stolen from my twitter account for your enjoyment/learning.
2) Combining two or more photo sets with handheld shooting
Creating a 3D model of an object without the background or pedestal (or whatever it is resting on) is more challenging. Let’s say you have a fossilized vertebra, and we need to photograph both the top and bottom bun. We need at least two sets of photos to accomplish this: one with the vertebra right side up and the other upside down (bottom bun on top). If you have the vertebra sitting right on a table, the table will be part of the model and seamlessly connected to the , so we need to elevate it a bit. If you put it on a plate, the same applies.
The idea behind the pedestal method: elevate the fossil above the table with a small pedestal so there is a gap between the table and the fossil. This prevents the camera from directly photographic where the fossil touches, and in this photoset, either the entire pedestal or the very top of it (provided you don't photograph from slightly below) will NOT be rendered in the point clouds. The side of the vertebra will be modeled well from both sets, but each end only from one set.
Pedestals. We need a pedestal that is quite a bit narrower than the burger. For a burger-sized fossil, a pedestal about 4-5cm high and wide is appropriate as it will keep the specimen from falling over. Pedestaled specimens fall over easily – which can cause 1) breakage and 2) failure of the photo set. Make sure it is stable! The key here is that with a pedestal, you must never have the pedestal contacting the fossil in view. If you photograph the sides of the burger, but at a slight downward angle, the bottom side of the lower bun is never in view and neither is the top of the pedestal. Therefore, as far as the computer is concerned, those points are not captured and therefore do not exist. Most critical is that there needs to be some sort of a surface that the computer can match points off of in BOTH photo sets. It is for this reason very difficult to apply the following method to something flat like a pancake (e.g. vertebral epiphysis), or very long like a rib.
Very important finding: clear pedestals, whether solid acrylic or a clear plastic cup, excel at this and become nearly invisible to the computer as the color/light values change at every angle, precluding the computer from matching many points at all. The clearer, the better!
The Tie Fighter method: use two different surfaces and two different pedestals to trick your dumb computer into only matching points on the fossil itself. The result will vaguely resemble the iconic Imperial Tie fighter from Star Wars. When the Dense Point cloud is done, the 'wings' can be easily deleted.
The Tie Fighter Method. When ready to photograph the other side, you must absolutely do three different things. 1) flip the specimen over. 2) use a different pedestal that looks different, so you aren’t accidentally matching both sides of the fossil to the same pedestal on one side. 3) Likewise, you need to set up a second area, ideally in a different room or different part of the room, to take the second set of photos using the second orientation. Further, if you need a third photo set, you’ll need a third location (and fourth, in rare cases where a fourth set is needed). Why is this necessary? If you shoot both orientations in the same location, EVEN IF YOU CHANGE THE BACKGROUND, the program will match points off on the walls and in the distance instead of the tabletop, and you’ll have a demented model in both orientations inside itself, and you’ll feel like calling a young priest and an old priest. True story: I switched the background for a walrus tusk once, using the tabletop and then adding a table cover, but in the photos, getting the side view of the tusk needed the camera almost down a few inches above the table, shooting almost horizontally. This resulted in the photos having the same stuff in the background aside from the tabletop, despite putting a tablecloth down and using different pedestals. What resulted were two tusks intersecting each other after photo alignment. The goal here is to trick the computer into thinking that the only parts it can match *any* points on are on the fossil and not anything else extraneous (like the same pedestal, or a stapler in the middle of the table if the photos are taken on either side of the stapler, or if both rooms have the same type of chair – not an uncommon problem if doing this in a college classroom).
Here's an example of the method in action: two photosets of the mandible of the toothed baleen whale Coronodon, photographed in two different rooms with different backgrounds, and since both sets include the mandible as the only uniquely shared object, the computer matched points successfully.
If you do this successfully, and if the fossil is rotated exactly 180 degrees, after photo alignment you will get two tabletop surfaces in the point cloud with the pedestal partially resolved, but disappearing close to the fossil, resembling a Tie Fighter from Star Wars, which is why I call this the Tie Fighter method. After the dense point cloud is done, simply select the tabletop and the pedestal ‘wings’ of the tie fighter and delete them – and you’ve got a complete point cloud for all surfaces. Make sure to add all the photos from both sets and run them as a single “chunk”.
Using the bullseye method for the top (Photoset 1) and bottom side (Photoset 2) of a skull. Using this method, the low ring from each set will be able to match points between sets. Therefore, the low ring of each photoset is the most critical and forms the ‘glue’ that sticks both photosets together.
Troubleshooting. If you try this, and only half the photos become aligned, there is not enough overlap between photo sets. Try re-shooting whatever set likely has the least coverage, delete those images from the folder and the file, and try again. It is for this reason that I generally leave the fossil in the same orientation as I photographed it in – if it hasn’t been moved, there’s a 50/50 shot that this set did not work, and I can just take some more photos that add to the deficient set (and then you don’t have to delete that set). If from the first set, however, you will never eyeball it close enough to get the fossil back into exactly the right orientation, so you’ll spend less time just taking the photos over again and deleting the deficient set.
If that still doesn’t work, you can try running them as different ‘chunks’. What this does is categorize subsets of the photos – lets say ‘vertebra right side up’ and ‘vertebra upside down’ as different chunks. Each chunk can be run independently, which sometimes can help. To put half the photos into a different chunk, select them on the photo pane and go right click-->move to new chunk. Do the photo alignment and dense point cloud steps, and then go to Workflow-->Align Chunks. There are a few variables you can mess around with, but I don’t have much advice about them, other than clicking preselection as ‘disabled’ rather than ‘generic’ seems to work more often. Make sure to check the boxes for each chunk you want (if only two, select both of them). Once done, go to Workflow-->Merge Chunks and this will stick them together. Merge the dense point clouds. But, here’s the rub: the pedestals may appear more connected to the models. So, what you’ve essentially done is a divided, time-intensive photo alignment. With the new merged chunk, all the cameras are now in the right spot! Now re-run the dense point cloud, and follow the other steps above as needed. If this still did not work, try different settings for chunk alignment. If that fails, start over and delete the photo sets (or, simply start a new folder).
An inexpensive light tent setup at the Mace Brown Museum of Natural History, sans turntable.
The Light Tent Method. I have not tried this, but I see setups that other people use, so I will attempt to describe it and how it could work, if you feel up to experimenting with it. The whole goal here is to trick the computer, and you can do that by either giving it one different background per orientation, or by making the background so featureless that it cannot possibly match any points on it at all. You can accomplish this by using a cake decorating turn table and put it inside of a thin fabric light tent diffuser. This method is useful for objects up to the size of a shoe. By placing lights on the other side of the light tent, it is extremely backlit, and should burn out into a nearly featureless single tone (the camera-savvy folks can also intentionally overexpose their photos to exaggerate this effect). The camera can be left on a tripod. For the next photo set, simply re-orient the fossil (use different objects between photo sets to prop it up if they are necessary) and shoot again – taking 40-50 photos per rotation. See more below for turntable photogrammetry.
Another way you could probably do this is to use a large light box, and a sheet of overhead projector plastic film, put the fossil on it, and just rotate the film around on the top of the lightbox; if the background is sufficiently burned out, it will achieve the same effect as the light tent method. Note: I used this as a way to get the background monotone so I could quickly delete the background from 2-3,000 photos for about a dozen different photos of earbones during my Ph.D.; I now realize that I could probably have just run the images through AGI photoscan without editing, and saved myself a near case of carpal tunnel during the final stages of Ph.D. work.
3) Creating 3D models of small fossils with a turntable and tripod.
What you’ll need: a cake decorating turntable, a macro lens, tripod, corded/remote shutter release, lamps, and three or four uniquely patterned sheets of paper without any images/patterns duplicated between. Also needed are three to four different pedestals. Pedestal options for small specimens: 1” sections of wooden dowels; clearish water bottle caps; a wooden dowel segment with three short wire prongs to ‘levitate’ a fossil up a centimeter or so above the dowel; a plastic lid to a small acrylic jewel case. A small wad of clay also works quite well. Be creative!
Simple turntable set up with a somewhat complicated pattern/image on a sheet of paper, tripod, DSLR camera with macro lens and remote shutter release, and two LED lamps for extra lighting. The image here happens to be an A4 size printout of a conference poster.
The set up: Place the fossil on its pedestal, and one of the four unique sheets of paper, onto the turntable. Elevate the camera on the tripod above the turntable, angled downward at a 45 degree angle. If a zoom lens, zoom in so that only the top of the turntable is in frame (this way, the rest of the set up does not matter and you don’t have to switch anything but the pedestal and sheet of paper). If a Macro lens, just move the tripod closer. The larger the fossil, the better the model!
Examples of a single photoset using a turntable: the photos on the left are a few of those from the actual set, with the camera/lens positioned as close as possible with the fossil still being in focus. This ensures that only the background (and not other objects on the table or stuff on the walls of the room) are being used to align the photos.
How to proceed: Take a photo of the fossil and spin the turntable 5 to 10 degrees (on a 12” wide turntable, that’s about a half inch along the outer edge or so… don’t double check my math, because I didn’t do any). That on average will give you somewhere around 50-70 photos per revolution. If you have a bunch of crevices or holes in frame, you can slow down and take double or triple the number of photos for part of the rotation (and alternatively, more sparsely for a big, bald, featureless part of a fossil). 50 photos per revolution is a pretty good rule of thumb. Now: switch out the background paper sheet, grab a different pedestal, and then put the fossil in a new orientation, and repeat. For the third set, switch em out again. A fourth set *may* be necessary, in which case, repeat these steps a fourth time. I’ve only ever used four using this method, and that seems sufficient to capture all the detail even on unusually complicated little bones like dolphin earbones. Run these all as a single chunk, and because the camera was kept stationary on the tripod, you will get three or four rings of photos shooting in sort of a cone around the fossil at that orientation! If one set fails, no need to delete: the photos are probably fine. Because you’re shooting down at an angle to avoid having anything off the turntable in frame, you may just need to add a couple more orientations and sets to get it to work; you can probably get away with a fourth set, or if needed, a fifth set but with 1/3 the photos. Ideally, you’ll get three sets of 50 photos each to work. The nice thing about this method is its speed: you can take all the photos you need in about 5 minutes (for all three sets). If the fossil falls over, find a better orientation and try again (or try the exact same thing and roll the dice). For this reason, it’s best to 1) find a cake turntable that doesn’t rattle or vibrate very much and 2) give it a test spin to see if it is stable on the pedestal.
Three photosets using three orientations and three different backgrounds – the result is a sparse point cloud with virtually the entire specimen being modeled, and each surface under the fossil is clear (parallel to the plane of the camera orbit). In the Dense Point Cloud, these can be easily deleted.
Speed run: Don’t bother looking through the lens while shooting. Make sure that the fossil is centered, and spin it around 360 degrees; if it goes out of frame at all, reposition it in the exact middle, or zoom out/move the tripod back. If it is within frame when you spin it around, there is no need to look through the viewfinder again: just have one hand on the turntable and the other on the shutter release. Take the photo, turn, photo, turn, etc. You may have to look in if it refuses to focus, but that doesn’t happen very often.
Fossil size: For this method, the smallest size is dictated by the size of the pedestal you can use, how much the turntable vibrates, and of course, how good your macro lens is. Most macro lenses have a functional limit to their focal depth and therefore past a certain size, the fossil will just appear smaller and you can’t get the camera any closer (and you also can’t zoom in, because it’s a macro lens). For my 100mm macro lens, the smallest I can get away with is about a 2 cm wide fossil or a smallish dolphin tooth.
What about maximum size? This is dictated by the size of the turntable and the orientation of the camera, and there are some tradeoffs. You can make the camera higher to keep the specimen completely in frame on the turntable only, but then the cone is tighter and there is less overlap between photo sets and it may fail more easily. You can include a little bit of the background, and make sure it’s monotone and featureless, and force the computer to match points on the turntable and the fossil (in which case you could also attempt the Light Tent Method, in which case you’re restricted to objects that can fit in the light tent and are not so heavy they damage the turntable). You can also make a larger turntable – either by putting a larger piece of cardboard on top and a larger patterned sheet of paper, or building your own turntable.
4) Scaling using Meshlab
When AGI photoscan makes your STL file, it uses arbitrary dimensions and it is typically, in my experience, somewhere between 1/10 and 1/50 actual size. To correct this, use a program called Meshlab (download it here). Once installed, go File > import mesh and browse to the correct STL file. Once the model is open, pick a few points you can reliably measure, and use the ‘measuring tape’ tool to measure the model digitally. Then, take the same measurement on the fossil with calipers (in millimeters). I find it is best to measure from broken points that are very clear and unambiguous, instead of typical anatomical measurements (e.g. transverse width). Now divide the actual caliper measurement by the digital measurement (the number should be greater than one, since the STL is always smaller than the actual fossil) – and that’s your correction factor (e.g. 34.65, meaning the fossil is 34 times bigger than the STL). Now, to be accurate, take three or four different measurements, calculate the correction factor, and take an average of the two that are closest to each other (discarding the others as outliers). Now, go Filters > Normals, Curvature, Orientation > Transform Scale and a window will pop up. Type the correction factor (e.g. 34.65) into the X,Y, and Z axis boxes, and hit apply - ONCE. The window does not go away after resizing, so if you *think* you need to click it again, you’ve just made it super gigantic, and will need to start over (reopen file, but you’ve already measured it, so go straight to transform scale). A little window saying “unify duplicate vertices” pops up, just click OK, I have no idea what it does, and the model will *probably* disappear. No worries, though: just go View > reset trackball and voila! It’s back, and at the proper scale. You can double check at this point and re-measure it with the measuring tape. Now, to save, go File > Export mesh as… and save as the same filename – saved now at the corrected size. Sometimes Meshlab seems like it needs to think for several minutes at this stage, but just check the folder and if the file is there with the correct time stamp (e.g. 30 seconds ago) then you can close Meshlab.
5) What about multielement specimens?
Sometimes you’ll have fossils that are large and broken and cannot be glued together. In which case, you’ll need a dense point cloud of part A, a separate one from part B, and a third photo set of parts A and B assembled together. Combine these into a single file as three different chunks – chunk A, chunk B, chunk AB – and try aligning and merging the chunks using the steps above. You can also load an existing .psz file into a second file, so if you’ve already done parts A and B months ago, no need to reinvent the wheel. Just make damn sure that when you take the combined AB set, you use a third location! For example: we have a giant dolphin skull curated in two pieces: a rostrum and braincase, which I photogrammetized a year ago separately. Months later, I took a third set of the two articulated properly – and literally just photographed it on its exhibit mount, and used the align chunks method and it turned out perfectly! After some computer wrangling, anyway.
6) What is the ultimate goal of all this, anyway?
I'm not going to lie. Many researchers treat 3D data and 3D visualization as a big sexy gimmick to wow people who have never seen a 3D model before and get grant money. "Look, we made a 3D model of this dino skeleton with LIDAR!" Okay cool, but why? In all seriousness, there are tons of grants going out to museums to help them digitize their collections, ostensibly to improve online outreach. This poses a couple of problems I'd like to bitch about for a minute. The first is that many museums need money for just regular cataloguing and curatorial supplies, and time and again I see museums that are financially well off getting money for 3D visualization instead of smaller, more destitute museums struggling to keep up. As a result, I find the abundance of these grants a bit offensive. A second problem is that a colleague of mine was (initially) turned down the opportunity to 3D scan (using a laser scanner) some critically important fossils (including type specimens, which should be accessible) at a prominent museum, because a 3D digitization campaign was being done in-house, and the fossils had already been scanned. The models were not shared by the museum, and they still aren't online, several years later (so they got scanned by my colleague anyway, who put them online).
So there are practical and logistical problems to these efforts. But why do them in the first place? I think there is clearly some merit to putting 3D models online, as they are much more tangible than photos, and many schools now have 3D printers. There are now entire lesson plans being put out through efforts like The Fossil Project around 3D printed megalodon teeth, and having school kids calculate the body length based on the size of the teeth. These sorts of applications will of course be biased against poor schools, but that's a different issue. We've been putting 3D models online through Sketchfab (see link above at top of article). That being said, my gut feeling is that the outreach aspect is being oversold. You'll notice that very few institutions have large libraries of paleontological 3D models: lip service for grants? I'm not sure. Photogrammetry is time consuming, but it's not *that* time consuming.
What do I find to be the most practical research application? Sharing anatomical information with colleagues on different continents. During my Ph.D., my adviser Dr. Ewan Fordyce and I decided to put 3D models of different eomysticetid whale earbones online so that our colleagues living on different continents wouldn't have to make an expensive trip to New Zealand to examine the specimens. We published STL and 3D pdfs of the earbones of Waharoa, Tohoraata, and the skull and earbones of Tokarahia on the University of Otago website. Now that I'm all grown up (aww) and am at my own institution with an abundance of spectacular fossils I am getting slowly published, it's my responsibility to keep that going so that some of my colleagues from far off places (New Zealand, Australia, Japan, Taiwan, Europe, and South America, to name a few) can examine the fossils in 3D from the comfort of their own office - or couch, given the current state of the world - and such 3D sharing of data is even more important in a year like 2020 where travel is so locked down.
Further Reading:
Mallison and Wings, 2014: A practical guide to photogrammetry. Journal of Paleontological techniques.
Heinrich Mallison's various blog posts on paleontological photogrammetry are listed below - these are extremely helpful, and go into some additional methods I've not experimented with, but I should re-read some of these and do some tinkering.
Photogrammetry 1: photogrammetry equipment
Photogrammetry 2: picture taking, general remarks
Photogrammetry 3: turntables
Photogrammetry 4: big bulky fossils
Photogrammetry 5: visual aid for turntables
Photogrammetry 6: building a model from the photos
Photogrammetry 7: multi chunk processing
Photogrammetry 8: scaling with hindsight
Photogrammetry 9: quick and dirty
Photogrammetry 10: improved method for mid-size objects
Photogrammetry 11: how to handle a project in photoscan
Photogrammetry 12: how to preserve strike/dip or other cardinal directions in a 3D model
Photogrammetry add on: the consequences of optimizing a sparse point cloud
Photogrammetry: speeding up the dense point cloud in photoscan
Hauf and Hendricks' guide to photogrammetry (and more detailed instructions of other functionality in AGI photoscan, including screenshots of the interface, menus, etc.) from Digital Atlas of Ancient Life.
Falkingham, 2012. Acquisition of high resolution 3D models using open source software. Palaeontologia Electronica.
Fahlke and Autenrieth 2016. Comparison of 3D models from micro CT and photogrammetry. Journal of Paleontological Techniques.













No comments:
Post a Comment