This year has seen a somewhat lower number of papers published on fossil marine mammals, myself included - though I'm quite pleased I finally got the paper on Valenictus sheperdi out. It's been a good year for fossil pinnipeds and cetaceans, especially from New Zealand - with the formal publication of the "Fossil Vertebrates of Southern Zealandia" edited volume. Additionally, a new edited volume on a single skeleton of the desmostylian Paleoparadoxia (or, at least a close relative) from Mizunami (Japan) also came out - though a formal taxonomic/morphological study was not included. As far as new taxa are concerned, there were two new genera of pinnipeds (both phocids) and four new species, six new genera and species of odontocetes, two new genera and species of mysticetes, and one new genus and species of archaeocete named this year.
[Abstract in Spanish] Los vertebrados fósiles de las líneas costeras marinas a lo largo de la costa atlántica de la provincia de Buenos Aires, en el este de Argentina, son bien conocidos desde el siglo XIX. A pesar de ser frecuentes a lo largo de la costa del área de Cabo San Antonio, la descripción de los vertebrados e invertebrados fósiles encontrados a lo largo de la costa permanece casi sin documentar y su descripción ha sido mayormente anecdótica. El objetivo de la presente contribución es el de describir una gran colección de fósiles encontrados a lo largo de la playa de varias localidades en el área del cabo de San Antonio. Los fósiles incluyen una mezcla de animales terrestres y marinos que probablemente pertenecen a la Formación Pozo 10 (aproximadamente 120.000 años antes del presente), correlacionable con el evento transgresivo marino conocido como "Belgranense". Varios fósiles sugieren aguas cálidas, reminiscentes de las que ocurren hoy en día en el norte de Brasil. Los fósiles aquí reportados son muy similares a los de los conjuntos de fósiles costeros encontrados en el sur de Brasil, Uruguay y el sur de la provincia de Buenos Aires, lo que sugiere algún tipo de continuidad faunística y ambiental a lo largo de las costas del sur durante el Pleistoceno medio.
Amane et al. Basilosauridae (Mammalia, Cetacea) from the Sahara Desert of southwestern Morocco.
The Sahara Desert in southwestern Morocco is today an important paleontological region for its richness of archaeocete whale remains from the middle and late Eocene. Remains of Basilosauridae have been reported from Gueran and El Breij of the Bartonian age and Ad-Dakhla of the Priabonian age. There are no complete skeletons, meaning it is rarely possible to associate the skull and the post-cranial bones directly. However, analysis of the size and shape of the recovered sequential vertebrae enables distinction and comparison of the species of basilosaurids. Lumbar vertebrae best distinguish the different species of Basilosauridae, and eight different species are present in the three localities combined. Reexamination of the characteristics of the vertebrae of the different species of basilosaurids allows a better expansion of knowledge concerning their taxonomy, stratigraphic distribution and paleogeography.
Bakker. Wrist and ankle bones of the walrus Odobenus rosmarus (Linnaeus, 1758).
This article discusses the characteristics of carpals and tarsals of the walrus Odobenus rosmarus (Linnaeus, 1758) and offers some notes on the fossil walrus Ontocetus emmonsi Leidy, 1859. The author investigated the bone characteristics on the basis of extant walrus bones and the extensive collection of fossil walrus material stored at the Natural History Museum Rotterdam. Identification sessions provided insights that may also be helpful to others. This article aims to contribute to better recognition and identification of the wrist and ankle bones of the walrus.
Several dolphin lineages have independently invaded freshwater systems. Among these, the evolution of the South Asian river dolphin Platanista and its relatives (Platanistidae) remains virtually unknown as fossils are scarce. Here, we describe Pebanista yacuruna gen. et sp. nov., a dolphin from the Miocene proto-Amazonia of Peru, recovered in phylogenies as the closest relative of Platanista. Morphological characters such as an elongated rostrum and large supraorbital crests, along with ecological interpretations, indicate that this odontocete was fully adapted to fresh waters. Pebanista constitutes the largest freshwater odontocete known, with an estimated body length of 3 meters, highlighting the ample resource availability and biotic diversity in the region, during the Early to Middle Miocene. The finding of Pebanista in proto-Amazonian layers attests that platanistids ventured into freshwater ecosystems not only in South Asia but also in South America, before the modern Amazon River dolphin, during a crucial moment for the Amazonian evolution.
Benites-Palomino et al. Trophic interacitons of sharks and crocodylians with a sea cow (Sirenia) from the Miocene of Venezuela.
Evidence of trophic interactions are not scarce in the fossil record, yet these are mostly represented by fragmentary fossils exhibiting marks of ambiguous significance. Differentiating between marks of active predation and scavenging events is therefore often challenging. Here, we report on a dugongine sea cow skeleton (partial skull and vertebrae) from the Lower to Middle Miocene Agua Clara Formation (Venezuela) with shark and crocodylian bite marks. The sirenian is identified as Culebratherium sp. and preserves crocodylian bite marks across the skeleton. The most conspicuous correspond to deep tooth impacts with dragging effect, concentrated in the rostrum of the specimen. We interpret these as the result of active predation because of the similarity with those produced when a crocodylian holds or rolls a prey. Additionally, shark bite marks can be observed throughout the skeleton, also evidenced by the finding of an isolated tiger shark (Galeocerdo aduncus) tooth associated with this skeleton. Because of the irregular distribution of the shark bite marks, these are interpreted as scavenging. Overall, these findings constitute one of the few records documenting multiple predators over a single prey, and as such provide a glimpse of the trophic networks during the Miocene in the region.
Bianucci et al. A new Late Miocene beaked whale (Cetacea, Odontoceti) from the Pisco Formation, and a revised age for the fossil Ziphiidae of Peru.
The previously scarce fossil record of Ziphiidae (beaked whales) has greatly increased recently thanks to the serendipitous discovery of high specimen concentrations along deep seafloors as well as to abundant inland finds from the Upper Miocene of the Pisco Formation (East Pisco Basin, Peru). In the latter unit, ziphiid remains are indeed among the most prevalent of the whole cetacean assemblage, being represented by four distinct genera and species plus at least two as-yet unnamed taxa. Here, we describe a fifth ziphiid genus and species from the Pisco strata, Mamaziphius reyesi n. gen. n. sp., based on a partial cranium from mid-Tortonian (lower Upper Miocene, 9.1-9.0 Ma) strata exposed at the locality of Cerros la Mama y la Hija. Though reminiscent of the extant genus Berardius, the holotype skull lacks two diagnostic characters of Berardiinae, namely, an isolated rounded protuberance formed by the interparietal or frontals on the posterior part of the vertex, and a posterior transverse narrowing of the nasals and frontals at the vertex. Our phylogenetic analysis reveals that Mamaziphius n. gen. is nested within the crown ziphiids, as sister group of the berardiines. In addition, we introduce two new clade names within Ziphiidae, namely, Messapicetiformes (for the so-called “Messapicetus clade”) and Vomeroziphii (for Ziphiinae + Hyperoodontinae and closely related forms). Another fragmentary specimen from the Pisco Formation is also briefly described herein. Furthermore, a comprehensive reappraisal of the geological age of the fossil beaked whales of Peru is provided based on new age calibrations, thus restricting the whole rich Peruvian record of this family (including the earliest-branching ziphiid, Ninoziphius platyrostris, which comes from Pisco-equivalent strata of the Sacaco area) to a Tortonian-Messinian interval younger than 9.10 Ma. No other inland unit worldwide preserves a record of fossil ziphiids as abundant, diverse and chronostratigraphically well-constrained as the Pisco Formation. In view of this, the absence of Vomeroziphii from the fossil content of the Pisco strata remains quite enigmatic.
A new fossil gray whale genus and species, Glaucobalaena inopinata, is established based on craniomandibular remains from the Pliocene Sabbie d’Asti Formation, Piedmont, northwestern Italy. The holotype (MGPT-PU 19512) consists of two cranial fragments corresponding to the posterolateral corners of the skull, including both partial periotics, and in the posterior portion of the right mandibular ramus preserving the condyle and angular process. The new taxon is characterized by gray whale (eschrichtiid) synapomorphies in the posterior portion of the mandible (dorsally raised mandibular condyle with articular surface faced dorsoposteriorly, well-developed and robust angular process of the mandible) and in the earbone (massive transverse elongation of the pars cochlearis, indistinct flange of the ventrolateral tuberosity, and triangular and short anterior process of the periotic). A CT scan of the cranial fragments allowed us to reconstruct tridimensional renderings of the periotic, revealing the dorsal morphology of this bone. A phylogenetic analysis confirmed the inclusion of Glaucobalaena inopinata within Eschrichtiidae (the family to whom gray whales are included) and showed that it is monophyletic with Gricetoides aurorae; our phylogenetic results show that Eschrichtioides gastaldii is the sister group of the genus Eschrichtius. Our work lends further support to the idea that Eschrichtiidae is a separate family of baleen whales, characterized by specialized ecomorphological characters evident in both skull and mandibular architecture.
Eomysticetidae are a clade of early diverging functionally toothless, longirostrine and likely baleen-bearing stem mysticete whales. Eomysticetid fossils are rare but known worldwide from Oligocene strata. The richest assemblage of eomysticetids has been uncovered in New Zealand from the Kokoamu Greensand and Otekaike Limestone (North Otago and South Canterbury regions, South Island). This includes some of the largest known eomysticetids, Tokarahia kauaeroa and Tokarahia lophocephalus, some older and more archaic forms such as Matapanui waihao, the fragmentary Tohoraata raekohao and Tohoraata waitakiensis, and the well-known Waharoa ruwhenua represented by several well-preserved skulls and mandibles of adults and juveniles. Studies of these New Zealand fossils strongly indicates monophyly of Eomysticetidae and suggest possible skim feeding behaviour, possession of non-functional teeth and baleen, extreme rostral lengthening during growth and peramorphic evolution, rostral kinesis, use of Zealandia as a calving ground, and probable extinction at or near the Oligo-Miocene boundary.
Remarks: A quick aside about this one. In spring 2023 I was drinking my morning coffee and had completely forgotten about the impending due date until I got an email from the editors informing me that the invited article was due in about three weeks. I had just gotten a flat tire, so I grabbed my laptop, pumped up the tire (hoping it wouldn't pop or leak too quickly), and drove down to Gerald's Tires. I had a two hour wait, and I cranked out ~90% of the text for this manuscript by the time the repairs were completed. Now, before you marvel at the speed at which I wrote it, bear in mind that it is a review article based mostly on my PhD thesis research - most PhDs can probably write a review of their own thesis material (hopefully from memory). Needless to say, we made the deadline!
Currently limited to cold climates near the Arctic circle, living walruses are the sole survivors of a previously much more diverse clade that occupied coastal waters throughout the northern hemisphere during the Mio–Pliocene. Though pinniped faunas have the highest diversity of walruses in the Miocene, the Purisima Formation of California records a moderately diverse assemblage of four walrus species. We report new specimens of tusked walruses (Odobeninae) including the oldest known members of Odobeninae, and Odobenini, and fossils of the specialized toothless odobenine walrus Valenictus Mitchell, 1961. Among these is the new species Valenictus sheperdi sp. nov., represented by a complete skull and referred post-crania from lower Pliocene strata within the Purisima Formation (5.33–4.89 Ma). Additionally, we report a geochronologically younger skull of Valenictus chulavistensis Deméré, 1994 from further up section (4.89–3.59 Ma). Expanded phylogenetic analysis recovers Odobeninae including Ontocetus Leidy, 1859 as the earliest diverging lineage in the Odobenini, and places a monophyletic Valenictus as the sister taxon to Pliopedia, Kellogg, 1921 which is included in a phylogeny for the first time; Odobenus is sister to the Valenictus + Pliopedia clade. Discovery of an isolated metacarpal near the base of the formation provides the oldest known well-dated evidence of odobenines. A diverse assemblage of molluskivores characterized the Neogene eastern North Pacific and their extinction around the Pliocene–Pleistocene boundary coincided with tectonically driven paleogeographic changes on the Pacific coast. The loss of temperate walruses may have provided opportunities for both new molluskivores and the otariid and phocid pinnipeds that make up present North Pacific pinniped communities.
Remarks: At last this study is published! Forrest Sheperd donated the specimen for study nearly 15 years ago, and I first started preparing it in 2010 at Museum of the Rockies. Completion of preparation had to wait until I returned from New Zealand, and I decided to try acid preparation - which worked surprisingly well! This is the most up to date study of fossil walruses.
Ontocetus is one of the most notable extinct odobenines owing to its global distribution in the Northern Hemisphere. Originating in the Late Miocene of the western North Pacific, this lineage quickly spread to the Atlantic Ocean during the Pliocene, with notable occurrences in England, Belgium, The Netherlands, Morocco and the eastern seaboard of the United States. Reassessment of a pair of mandibles from the Lower Pleistocene of Norwich (United Kingdom) and a mandible from the Upper Pliocene of Antwerp (Belgium) that were referred to as Ontocetus emmonsi reveals existences of features of both Ontocetus and Odobenus. The presence of four post-canine teeth, a lower canine larger than the cheek-teeth and a lower incisor confirms the assignment to Ontocetus; simultaneously, characteristics such as a fused and short mandibular symphysis, a well-curved mandibular arch and thin septa between teeth align with traits usually found in Odobenus. Based on a combination of these characters, we describe Ontocetus posti, sp. nov. Its mandibular anatomy suggests, a better adaptation to suction-feeding than what was previously described in the genus suggesting that Ontocetus posti sp. nov. likely occupied a similar ecological niche to the extant walrus Odobenus rosmarus. Originating from the North Pacific Ocean, Ontocetus most likely dispersed via the Central American Seaway. Although initially discovered in the Lower Pliocene deposits of the western North Atlantic, Ontocetus also left its imprint in the North Sea basin and Moroccan Plio-Pleistocene deposits. The closure of the Isthmus of Panama during the Mio-Pliocene boundary significantly impacted the contemporary climate, inducing global cooling. This event constrained Ontocetus posti in the North Sea basin leaving the taxon unable to endure the abrupt climate changes of the Early Pleistocene, ultimately going extinct before the arrival of the extant counterpart, Odobenus rosmarus.
Kekenodontids are the only known archaeocetes (stem cetaceans) from the late Oligocene. They possess a unique combination of morphological features seen in both more primitive Eocene basilosaurid archaeocetes and more derived Neoceti (mysticetes and odontocetes). However, much remains unknown about the clade, including its acoustic biology. Based on its phylogenetic position crownward to basilosaurids as the latest-diverging archaeocete, we hypothesize that kekenodontids would be specialized for hearing low-frequency sounds. Here, we provide the first report on the cochlear anatomy of a kekenodontid using the holotype of Kekenodon onamata from New Zealand. We compare the cochlear morphology of K. onamata to a sample of extinct and extant cetaceans and quantify shape differences using three-dimensional geometric morphometrics. The analyses show that K. onamata was indeed adapted to hear low frequencies and suggests low-frequency hearing may be a characteristic of raptorial macrophagous fossil cetaceans in contrast to infrasonic bulk filter-feeding mysticetes and ultrasonic echolocating odontocetes.
The Kekenodontidae are late-surviving archaeocetes from the Late
Oligocene of Southwest Pacific that includes a single-named species, Kekenodon onamata. Tohoraonepu nihokaiwaiu
is a new genus and species of small body-sized kekenodontid from the
upper Oligocene (Chattian) Kokoamu Greensand of Otago, South Island, New
Zealand. Phylogenetic analyses recover T. nihokaiwaiu
within a monophyletic Kekenodontidae, forming a clade with an unnamed
provisional kekenodontid, OU 22023. Kekenodontids are recovered
crownward to basilosaurids and stemward to a paraphyletic group of
toothed ‘mysticetes’ that are excluded from Neoceti. The analyses
confirm the identification of kekenodontids as the latest-diverging
archaeocetes that persisted into the Late Oligocene. The holotype OU
22394 is a juvenile individual preserving several isolated heterodont
teeth with characteristics of deciduous teeth, including unmineralized
pulp cavities and cheek teeth with lower-lying triangular crowns that
are different from all known kekenodontids. Diphyodonty is known from
Eocene archaeocetes but is unknown from geologically younger toothed
cetaceans, with monophyodonty being hypothesised for all Neoceti.
Inferences of diphyodonty in T. nihokaiwaiu would be the
first instance in Cetacea from rocks geologically younger than the
Eocene and would indicate diphyodonty persisted in some Late Oligocene
archaeocetes.
The repeated returns of vertebrates to the marine ecosystems since the Triassic serve as an evolutionary model to understand macroevolutionary change. Here we investigate the effects of the land-to-sea transition on disparity and constraint of the vertebral column in aquatic carnivorans (Carnivora; Pinnipedia) to assess how their functional diversity and evolutionary innovations influenced major radiations of crown pinnipeds. We use three-dimensional geometric morphometrics and multivariate analysis for high-dimensional data under a phylogenetic framework to quantify vertebral size and shape in living and extinct pinnipeds. Our analysis demonstrates an important shift in vertebral column evolution by 10–12 million years ago, from an unconstrained to a constrained evolutionary scenario, a point of time that coincides with the major radiation of crown pinnipeds. Moreover, we also demonstrate that the axial skeleton of phocids and otariids followed a different path of morphological evolution that was probably driven by their specialized locomotor strategies. Despite this, we found a significant effect of habitat preference (coastal versus pelagic) on vertebral morphology of crown taxa regardless of the family they belong. In summary, our analysis provides insights into how the land-to-sea transition influenced the complex evolutionary history of pinniped vertebral morphology.
The Natural History Museum of the University of Pisa hosts the most important osteological collection of extant cetaceans in Italy as well as one of the most relevant all over Europe. Furthermore, it also preserves a significant palaeontological collection that includes several holotypes and otherwise unique specimens of Archaeoceti (archaic cetaceans), Mysticeti (baleen whales) and Odontoceti (toothed whales). Here, we provide a historical overview of these collections and the corresponding displays, with special attention paid to the origin, development and design of the ‘Archaeocete Hall’ and ‘Cetacean Gallery’. These comprise what may be the largest exhibition worldwide among those dedicated exclusively to cetaceans—one that includes 28 complete skeletons and one skull belonging to 27 extant species as well as fossils of nine extinct species. Our review also reveals that the museum exhibitions feature the oldest known specimen of Mesoplodon bowdoini and the type specimen of Ziphius savi, the latter being a validly described species that is currently regarded as a junior synonym of Ziphius cavirostris. Also significant is the display of several holotype specimens of fossil species such as the protocetid archaeocete Aegyptocetus tarfa, the balaenid baleen whales Balaena montalionis and Balaenula astensis, and the monodontid Casatia thermophila. The Archaeocete Hall and Cetacean Gallery are highly appreciated by visitors as well as perused by the museum’s educational team. The online archiving of 3D models of many of the MSNUP specimens on the open-access digital repository Sketchfab and their subsequent dissemination through the Wikimedia platforms has led to the creation of a major osteological resource—one that is broadly accessible to internet users worldwide.
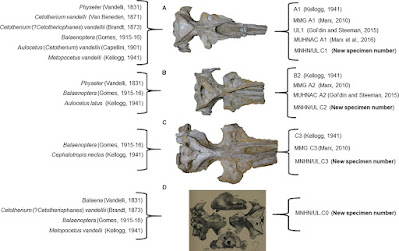
Cetotheriidae is a family of baleen whales that went nearly extinct during the Pleistocene (excluding Caperea marginata). For a long time, the Cetotheriidae family has been seen as a problematic clade, but in the past two decades there have been various studies trying to resolve the phylogeny of this group. In 1831, Alexandre Vandelli described three cetotheriid skulls, found during a gold exploration at Adiça beach (Portugal). These specimens constituted the first Portuguese vertebrate fossils ever published in the literature. Another skull was added to the “Vandelli skulls” by Jacinto Pedro Gomes, in 1914, during a survey of the Museu Nacional de História Natural collections without giving information on the origin of this skull. In 1941, Remington Kellogg states that one of the original “Vandelli skulls” is no longer present in the Museu Nacional de História Natural collections. Until today, there is no information on how, or exactly when, the fourth skull and one of the original three “Vandelli skulls” appeared and disappeared, respectively. Since their discovery, all the attempts to describe these specimens were not based on direct observations and no comprehensive phylogenetic analysis have included the three skulls. Here we provide a detailed anatomic description, a new phylogenetic analysis and a palaeoecological reconstruction of these specimens, clarifying their relationships within the Cetotheriidae family and fostering the importance of these historical specimens to the modern comprehension of fossil whale evolution. In addition, our results support that Cephalotropis nectus is a valid species with an emended diagnosis. We also concluded that two specimens belong to a new genus, forming two new fossil species (new combinations).
We describe and analyze a rib fragment of a small cetacean from the Castell'Arquato Plio-Pleistocene Basin (Northern Apennines, Italy) that displays various tooth marks featuring parallel striations similar to those left by the serrated tooth of the extant white shark, Carcharodon carcharias. The discovery locality, known as "Buca della Balena", was an inner-shelf marine setting where sharks may have scavenged on drifting cetacean carcasses in Piacenzian times. The high number of marks found on the small bone fragment suggests multiple bites by one or more shark individuals. The rib fragment studied is one of the few osteological specimens from the Pliocene of the Mediterranean Basin to preserve white shark tooth marks.
Body size is of fundamental importance to our understanding of extinct
organisms. Physiology, ecology and life history are all strongly
influenced by body size and shape, which ultimately determine how a
species interacts with its environment. Reconstruction of body size and
form in extinct animals provides insight into the dynamics underlying
community composition and faunal turnover in past ecosystems and broad
macroevolutionary trends. Many extinct animals are known only from
incomplete remains, necessitating the use of anatomical proxies to
reconstruct body size and form. Numerous limitations affecting the
appropriateness of these proxies are often overlooked, leading to
controversy and downstream inaccuracies in studies for which
reconstructions represent key input data. In this perspective, we
discuss four prominent case studies (Dunkleosteus, Helicoprion, Megalodon and Perucetus)
in which proxy taxa have been used to estimate body size and shape from
fragmentary remains. We synthesise the results of these and other
studies to discuss nuances affecting the validity of taxon selection
when reconstructing extinct organisms, as well as mitigation measures
that can ensure the selection of the most appropriate proxy. We argue
that these precautionary measures are necessary to maximise the
robustness of reconstructions in extinct taxa for better evolutionary
and ecological inferences.
Godfrey et al. First report in the fossil record of a shark tooth embedded in a pinniped bone.
There are now many examples in the fossil record of shark bite marks
preserved on biogenic materials including coprolites, ammonoids, sea
star ossicles, an echinoid, and bone and calcified cartilage. These
exceptional fossils document evidence of exploratory behavior, active
predation, and/or scavenging. However, only a small subset report on the
presence of shark teeth embedded in fossilized bone or cartilage.
Although a few shark tooth-marked seal bones are known from the fossil
record, no direct evidence of predation or scavenging in the form of a
shark tooth embedded in a fossil seal bone has yet been documented.
Herein, we describe the first shark tooth embedded in a seal (Phocidae
Gray, 1821) bone, a calcaneum (CMM-V-6964), that was surface collected
in Mosaic's South Fort Meade Mine, Hardee County, Fort Meade, Florida,
United States. The isolated bone originated from within the Bone Valley
Member of the Peace River Formation (Hawthorn Group). The partial tooth
is identified as having come from an Early Pliocene great white shark,
Carcharodon carcharias (Linnaeus, 1758). This discovery also represents
the first C. carcharias tooth ever found embedded in a fossil bone. The
embedded tooth may have come about as a result of active predation or
scavenging. The extant macropredatory sharks, Carcharodon carcharias
(great white shark), Notorynchus cepedianus (Péron, 1807) (sevengill
shark), Somniosus microcephalus (Bloch & Schneider, 1801) (the
Greenland shark), and Somniosus antarcticus Whitley, 1939 (sleeper
shark), are known to actively prey upon seals (Pinnipedia Illiger,
1811). If this peculiar fossil association resulted from active
predation, the seal did not survive the encounter because there is no
evidence of healing in the area around the embedded shark tooth.
Hafed et al. New Neogene fossil phocid postcranial material from the Atlantic (USA).
To date, the phocid fossil record from the Western Atlantic includes representatives of the subfamilies Monachinae and Phocinae. New partial postcranial bones (humerus, innominate) of true seals from the Meherrin River, Murfreesboro, Hertford County, North Carolina, U.S.A. are morphologically examined in detail. The newly discovered postcranial bones are compared to representatives of other pinnipeds (Phocidae, Otariidae, and Odobenidae) and to representatives from other phocid subfamilies (Cystophorinae, Devinophocinae, Monachinae, and Phocinae). Detailed morphological analyses demonstrate characteristics that are known to be phocid-specific, such as: the humeral deltoid crest extending to half its length, becoming thinner towards the distal portion; short and flattened femur, with a diaphysis that is broader at its distal end; and absence of femoral lesser trochanter. Taxonomic assessment identified the humerus as Phocanella pumila (Subfamily Phocinae), and the three innominate bones (Subfamily Monachinae): Homiphoca capensis, Sarcodectes magnus, and a newly discovered genus and species Seronectes meherrinensis. In the same collection, a fossil femur from the Chesapeake Group, St. Marys Formation, Maryland-Virginia is identified as Homiphoca capensis (Subfamily Phocinae). This study further confirms the likely presence of adaptively diverse phocids from either the subfamily Monachinae or Phocinae in the same locality during the Miocene time.
Despite two centuries of fossils collecting, no cetacean remains from the Oligocene marine deposits of the Mainz Basin (western Germany) have ever been reported. Here, we describe a possible mysticete tooth from the sand pit of Eckelsheim, which exposes high energy deposits belonging to the Rupelian Alzey Formation. The latter has yielded a rich assemblage of vertebrates and invertebrates, but so far, only one marine mammal in the form of the sirenian Kaupitherium. The whale tooth in some ways resembles the m2 of Llanocetus from the latest Eocene of Seymour Island, Antarctica. If the find from the Mainz Basin is not a regionally evolved form, Llanocetus, which is known from the South Atlantic, could have migrated through the Atlantic realm during the early Oligocene. It cannot be excluded that the tooth represents a more widely occurring lineage, neither endemic nor necessarily related to llanocetids, that—given the generally poor Rupelian record—has not been well documented yet.
Kimura and Hasegawa. New fossil lipotid (Cetacea, Delphinida) from the Upper Miocene of Japan
Two specimens of fossil lipotid have been recovered from the Upper Miocene of Gunma and Tochigi prefectures, Japan. The specimens consist of crania including periotic and tympanic bullae. We describe and diagnose the specimens as a new genus and species of the family Lipotidae, Eolipotes japonicus, gen. et sp. nov. The paratype of E. japonicus, GMNH-PV-1011, was found stratigraphically between two tuff layers and a well constrained age interval (11.29–11.25 Ma) can be proposed, indicating that E. japonicus is the oldest precisely dated lipotid species yet described. Phylogenetic analysis revealed that E. japonicus is more closely related to Parapontoporia spp. than to the recently extinct Yangtze river dolphin, Lipotes vexillifer. Given the phylogenetic relationships and parsimonious distribution of the event to fluvial invasion in Lipotidae, it is suggested that at least two independent invasions to the freshwater habitat occurred in Lipotidae.
Kimura and Hasegawa. A new species of Late Miocene balaenopterid, Incakujira fordycei, from Sacaco, Peru. [links directly to pdf download]
We describe a new specimen of the Balaenopteridae from the upper Miocene, Pisco Formation, Peru. The holotype specimen is nearly a complete skeleton and most of the bones are preserved in articulated. Moreover, the holotype skull retains a rack of baleen in near-life position. Here we refer this specimen as a new species of the genus Incakujira, I. fordycei sp. nov. This new specimen will expand our knowledge of the paleobiodiversity and disparity of the balaenopterids. The vertebral and baleen plate morphology imply that I. fordycei was an agile swimmer with feeding on variety of diets (evasive and also non-evasive prey items).
(The paper doesn't have any figures, but here's a lovely photo of Carolina, Daphne Lee, and Jefferey Robinson, the three authors, with the edited volume in the Otago Geology Museum - and parts of the Nihoroa and ?Aureia holotype skulls out for viewing - photo by Otago Daily Times).
Loch et al. Fossil vertebrates from southern Zealandia: taonga of international significance.
Vertebrate fossils from Aotearoa New Zealand have revealed the global significance of Zealandia on the origins of modern birds, the history of cetaceans during major climatic events of the Cenozoic and the evolution of cartilaginous and ray-finned fish. Internationally important collections of vertebrate fossils are housed in collections across Aotearoa and have attracted researchers from around the world studying evolution, biogeography and climate change. This special issue of the Journal of the Royal Society of New Zealand celebrates the vertebrate fossils of Aotearoa by showcasing taonga (treasures) that are significant to global and local vertebrate history.
Apicobasal ridges are longitudinal ridges of enamel that are particularly common in several clades of aquatic-feeding predatory amniotes, including Plesiosauria, Ichthyosauria, Mosasauridae, Crocodylia, and Spinosauridae, as well as some early members of Cetacea. Although the repeated evolution of these dental ridges in unrelated clades suggests an adaptive benefit, their primary function in feeding is debated. Hypothesized functions range from increasing tooth strength to improving prey puncture or removal efficiency, but these have never been quantitatively tested. This study utilizes finite element analysis (FEA) to assess the impact of apicobasal ridges upon tooth crown strength in aquatic-feeding amniotes. Drawing on morphometric data from fossilized tooth crowns, a set of digital models was constructed to calculate the performance of smooth and ridged tooth variants under simulated bite force loadings. The similarities in overall stress distribution patterns across models of the same tooth shape, regardless of the presence or morphology of ridges, indicate that apicobasal ridges have little impact on stress reduction within the tooth crown. Ultimately, these findings suggest that apicobasal ridges have a minimal role in improving crown strength and form a framework for future research into the remaining hypotheses.
Martinez et al. Controls on late Miocene marine vertebrate bonebed genesis in Northern Chile.
The Bahía Inglesa Formation in the Atacama Region of Chile (27°S) hosts
one the world's best-preserved Cenozoic marine vertebrate death
assemblages attributed to harmful algal bloom (HAB)-mediated mass
mortalities. However, the lack of a well-dated depositional model
prevents understanding of the timing of fossil accumulation and
associated paleoenvironmental conditions. We present a revised
chronostratigraphic framework for the Bahía Inglesa Formation at the
Cerro Ballena and Mina Fosforita paleontological sites based on detailed
field sedimentology, paleontology, and U Pb
zircon and phosphate geochronology. We integrate maximum depositional
ages (MDAs) of intercalated volcanic ash and sandstone beds with
published basinal chronostratigraphy to provide the first correlation
between both localities. Detrital zircon U
Pb
zircon and phosphate geochronology. We integrate maximum depositional
ages (MDAs) of intercalated volcanic ash and sandstone beds with
published basinal chronostratigraphy to provide the first correlation
between both localities. Detrital zircon U Pb
ages suggest that the highly articulated cetacean carcasses preserved
at Cerro Ballena were rapidly deposited in a barrier-protected shoreface
environment at ca. 6.1 Ma during periods of voluminous volcanic ash and
diatom accumulation on the shelf. Evidence of disarticulated and
taxonomically diverse vertebrate fossils within the Mina Fosforita
bonebed suggest deposition in an unsheltered offshore transition to
inner shelf environment with strong wave action and rapid flooding
conditions between ca. 7.4 and 6.8 Ma. We propose that the fossil
accumulations at Cerro Ballena and Mina Fosforita were deposited during
distinct sedimentation events controlled by local bay paleogeography and
rapidly fluctuating relative sea-level conditions. We compare our
chronostratigraphic results with 2969 published magmatic ages from the
Central Andes and propose a correlation between the mass deaths of
marine mammals in northern Chile and intensifications in volcanism,
ocean fertilization, phosphogenesis, and HAB blooms during the late
Miocene.
Pb
ages suggest that the highly articulated cetacean carcasses preserved
at Cerro Ballena were rapidly deposited in a barrier-protected shoreface
environment at ca. 6.1 Ma during periods of voluminous volcanic ash and
diatom accumulation on the shelf. Evidence of disarticulated and
taxonomically diverse vertebrate fossils within the Mina Fosforita
bonebed suggest deposition in an unsheltered offshore transition to
inner shelf environment with strong wave action and rapid flooding
conditions between ca. 7.4 and 6.8 Ma. We propose that the fossil
accumulations at Cerro Ballena and Mina Fosforita were deposited during
distinct sedimentation events controlled by local bay paleogeography and
rapidly fluctuating relative sea-level conditions. We compare our
chronostratigraphic results with 2969 published magmatic ages from the
Central Andes and propose a correlation between the mass deaths of
marine mammals in northern Chile and intensifications in volcanism,
ocean fertilization, phosphogenesis, and HAB blooms during the late
Miocene.
Marx et al. Strontium isotopes reveal a globally unique assemblage of Early Miocene baleen whales.
The earliest Miocene (Aquitanian, 23–20 Ma) remains a critically under-sampled ‘dark age’ in cetacean evolution. This is especially true of baleen whales (mysticetes), Aquitanian specimens of which remain almost entirely unknown. Across the globe, the nature of the cetacean fossil record radically shifts at the Oligocene-Miocene boundary, with mysticetes and some archaic odontocete lineages suddenly disappearing despite the availability of cetacean-bearing rock units. New Zealand is the only place worldwide where this change is not readily apparent, with baleen whales apparently persisting into the earliest Miocene. Whether this is a genuine pattern has so far remained obscured by a lack of biostratigraphic resolution associated with the Oligo-Miocene boundary. Here, we report 23 new strontium (87Sr/86Sr) dates from Lentipecten shells associated with 16 mysticete and seven odontocete specimens, respectively. Of these, eight fall within the Early Miocene and seven – including five mysticetes – specifically within the Aquitanian. Our findings confirm the unique nature and global importance of the cetacean fossil record from New Zealand, and provide a foundation for investigations into the causes and effects of the Early Miocene cetacean ‘dark age’.
Meekin et al. Aureia rerehua, a new platanistoid dolphin from the Oligocene of New Zealand with a unique feeding method.
Pre-Miocene, stem odontocetes are known for their procumbent incisors and their function has been the subject of much speculation. Notable among these were Waipatia and several related taxa from New Zealand. Though some studies hypothesise the function of these teeth was for thrusting, the here described Aureia rerehua has unique teeth which might have formed a cage around small fish. These teeth, along with a weak vertex, flexible neck, and the smallest size among its relatives would make it a capable hunter in shallow waters. The addition of A. rerehua along with other taxa to phylogenetic analyses show three broad groups within taxa related to Waipatia and Otekaikea based on the divergence of the function of their teeth and the possible feeding strategies employed to catch prey.
Pacific Walruses (Odobenus rosmarus divergens [Illiger 1815]) are gregarious marine mammals considered to be sentinels of the Arctic because of their dependence on sea ice for feeding, molting, and parturition. Like many other marine mammal species, their population sizes were decimated by historical overhunting in the nineteenth and twentieth centuries. Although they have since been protected from nearly all commercial hunting pressure, they now face rapidly accelerating habitat loss as global warming reduces the extent of summer sea ice in the Arctic. To investigate how genetic variation was impacted by overhunting, we obtained mitochondrial DNA sequences from historic Pacific Walrus samples in Alaska that predate the period of overhunting, as well as from extant populations. We found that genetic variation was unchanged over this period, suggesting Pacific Walruses are resilient to genetic attrition in response to reduced population size, and that this may be related to their high vagility and lack of population structure. Although Pacific Walruses will almost certainly continue to decline in number as the planet warms and summer sea ice is further reduced, they may be less susceptible to the ratcheting effects of inbreeding that typically accompany shrinking populations.
Extremes in organismal size have broad interest in ecology and evolution because organismal size dictates many traits of an organism’s biology. There is particular fascination with identifying upper size extremes in the largest vertebrates, given the challenges and difficulties of measuring extant and extinct candidates for the largest animal of all time, such as whales, terrestrial non-avian dinosaurs, and extinct marine reptiles. The discovery of Perucetus colossus, a giant basilosaurid whale from the Eocene of Peru, challenged many assumptions about organismal extremes based on reconstructions of its body weight that exceeded reported values for blue whales (Balaenoptera musculus). Here we present an examination of a series of factors and methodological approaches to assess reconstructing body weight in Perucetus, including: data sources from large extant cetaceans; fitting published body mass estimates to body outlines; testing the assumption of isometry between skeletal and body masses, even with extrapolation; examining the role of pachyostosis in body mass reconstructions; addressing method-dependent error rates; and comparing Perucetus with known physiological and ecological limits for living whales, and Eocene oceanic productivity. We conclude that Perucetus did not exceed the body mass of today’s blue whales. Depending on assumptions and methods, we estimate that Perucetus weighed 60–70 tons assuming a length 17 m. We calculated larger estimates potentially as much as 98–114 tons at 20 m in length, which is far less than the direct records of blue whale weights, or the 270 ton estimates that we calculated for body weights of the largest blue whales measured by length.
So far, 41 sirenian fossil records have been known in Japan. Most of
them belong to the subfamily Hydrodamalinae and the fossil localities
are mainly distributed from eastern Honshu to Hokkaido. The
Hydrodamalinae is the only cold-adapted taxon among sirenians. A new
fossil was found from the Late Pliocene Zukawa Formation in Takaoka,
Toyama Prefecture. It is the first record of sirenian fossil in Toyama
Prefecture and its locality is close to the southwesternmost of fossil
distribution of Hydrodamalis in the Sea of Japan side. Collected
fossils are partial bones of skull, scapula, humerus, vertebrae and
ribs, and they are presumed to be a same individual because of no
overlapping parts in the skeleton. The fossil is identified as the genus
Hydrodamalis (Hydrodamalinae) based on several distinct features
of the skull and considered to be a young individual with loose bone
sutures. Furthermore, some unique morphological features in temporal,
mandible and humerus are different from recognized species of Hydrodamalis.
The fossil age is estimated to be around 2.7 Ma, because assumed fossil
bearing bed is about 6 meters upper from the Datum A. The Datum A is a
boundary of changing to cold assemblage in calcareous nannofossils and
its age estimated to be 2.75 Ma. The fossil is important on the
paleobiogeography of Hydrodamalis, because the fossil shows
clearly that sirenianʼs southward movement and climate cooling are
closely related in the Sea of Japan side. So the latest Pliocene climate
of Toyama area was suitable for Hydrodamalis. In this way, cold-adapted Hydrodamalis
may have migrated southward intermittently from the northern area to
Honshu during the Pliocene to Pleistocene in response to cooling climate
change.
Nowadays, the infraorder Delphinida (oceanic dolphins and kin) represents the most diverse extant clade of Cetacea, with delphinids alone accounting for more than 40% of the total number of living cetacean species. As for other cetacean groups, the Early Miocene represents a key interval for the evolutionary history of Delphinida, as it was during this time span that the delphinidans became broadly distributed worldwide, first and foremost with the widespread genus Kentriodon and closely related forms. Here, we report on a new odontocete find from Burdigalian (20.4–19.0 Ma) deposits of the Friulian-Venetian Basin of northeastern Italy, consisting of the partial cranium of a small delphinidan with associated ear bones (right periotic, stapes, malleus and tympanic bulla). Osteoanatomical considerations and comparisons allow us to assign the studied specimen to the genus Kentriodon. This is the first confirmed record of Kentriodon from Europe as well as from the whole proto-Mediterranean region. Stratigraphic and phylogenetic considerations suggest that our new specimen may represent the geologically oldest member of Kentriodon. The evolutionary success of Kentriodon may correlate with the emergence of narrow-band high-frequency echolocation as a possible strategy to escape acoustic detection by large marine predators such as the squalodontids. In addition, the relatively high encephalization quotient of Kentriodon spp. may have provided these early dolphins with some kind of competitive advantage over the coeval non-delphinidan odontocetes.
Pinnipeds (seals, sea lions, walruses, and their fossil relatives) are one of the most successful mammalian clades to live in the oceans. Despite a well-resolved molecular phylogeny and a global fossil record, a complete understanding of their macroevolutionary dynamics remains hampered by a lack of formal analyses that combine these 2 rich sources of information. We used a meta-analytic approach to infer the most densely sampled pinniped phylogeny to date (36 recent and 93 fossil taxa) and used phylogenetic paleobiological methods to study their diversification dynamics and biogeographic history. Pinnipeds mostly diversified at constant rates. Walruses, however, experienced rapid turnover in which extinction rates ultimately exceeded speciation rates from 12 to 6 Ma, possibly due to changing sea levels and/or competition with otariids (eared seals). Historical biogeographic analyses, including fossil data, allowed us to confidently identify the North Pacific and the North Atlantic (plus or minus Paratethys) as the ancestral ranges of Otarioidea (eared seals + walrus) and crown phocids (earless seals), respectively. Yet, despite the novel addition of stem pan-pinniped taxa, the region of origin for Pan-Pinnipedia remained ambiguous. These results suggest further avenues of study in pinnipeds and provide a framework for investigating other groups with substantial extinct and extant diversity.
The raoellid artiodactyl Indohyus indirae is known from northern Pakistan and northwestern India, with substantial skeletal material found in the Sindkhatudi locality near Kalakot in Kashmir. Eight specimens from this locality, two of which exhibit deciduous dentitions, offer invaluable insights into cranial osteology of Indohyus indirae. We present a comprehensive examination of the cranial osteology of Indohyus, highlighting detailed unique features using this material. These specimens, though flattened dorsoventrally and mediolaterally, allow for anatomical observations. Previous literature has extensively employed these specimens for phylogenetic analyses indicating that Indohyus is a close relative of cetaceans. These findings are partly based on the material described here. Notably, the cranial morphology of Indohyus differs in several aspects from that of most early and middle Eocene artiodactyls. For instance, the rostrum of Indohyus is longer than that of most middle Eocene artiodactyls, resembling Eocene cetaceans. The positioning of the nasal opening above the incisors is similar to terrestrial artiodactyls. However, the ventral wall of the nasopharyngeal duct does not extend to the ear region, which distinguishes it from Eocene cetaceans. Moreover, the frontal bone is thick and has a concave profile, forming a dorsal shield for the laterally facing orbits. Furthermore, the tympanic bone has an involucrum, a feature that characterizes all cetaceans. These cranial features may be indicative of specific cranial functions and, given their similarity in some regards to Eocene cetaceans, could be related to the land-to-water transition. Further research, including explicit functional studies, is required to investigate these hypotheses.
[No abstract, introduction included here instead]. The holotype, and only known specimen, of Afrophoca libyca Koretsky & Domning, 2014, is a right mandible fragment (ELNRP 2Z131) extending from the posterior wall of the canine alveolus mesially to the posterior alveolus of the m1 distally. It retains almost complete but damaged p3 and p4 and the anterior root of the m1. The specimen is from Gebel Zelten (also known as Jabal Zaltan), Libya, a basal Middle Miocene locality (Koretsky and Domning 2014). Afrophoca libyca has been interpreted by these authors as the oldest known representative of the family Phocidae in the Old World, and included in the subfamily Monachinae. However, examination of the fossil reveals that it belongs, in fact, to the medium-sized anthracothere, Afromeryx zelteni, Pickford, 1991, of which the type locality is also Gebel Zelten. The attribution of the holotype and only known specimen of Afrophoca libyca to Afromeryx zelteni impacts on recent literature that accepted the original identification of Koretsky and Domning (2014) rendering the biogeographic and biochronological scenarios obsolete, and the phylogenetic analyses of no relevance for understanding the origins of Phocidae.
Pimiento et al. The extinct marine megafauna of the Phanerozoic.
The modern marine megafauna is known to play important ecological roles
and includes many charismatic species that have drawn the attention of
both the scientific community and the public. However, the extinct
marine megafauna has never been assessed as a whole, nor has it been
defined in deep time. Here, we review the literature to define and list
the species that constitute the extinct
marine megafauna, and to explore biological and ecological patterns
throughout the Phanerozoic. We propose a size cut-off of 1 m of length
to define the extinct marine megafauna. Based on this definition, we
list 706 taxa belonging to eight main groups. We found that the extinct
marine megafauna was conspicuous over the Phanerozoic and ubiquitous
across all geological eras and periods, with the Mesozoic, especially
the Cretaceous, having the greatest number of taxa. Marine reptiles
include the largest size recorded (21 m; Shonisaurus sikanniensis)
and contain the highest number of extinct marine megafaunal taxa. This
contrasts with today’s assemblage, where marine animals achieve sizes of
>30 m. The extinct marine megafaunal taxa were found to be
well-represented in the Paleobiology Database, but not better sampled
than their smaller counterparts. Among the extinct marine megafauna,
there appears to be an overall increase in body size through time. Most
extinct megafaunal taxa were inferred to be macropredators
preferentially living in coastal environments. Across the Phanerozoic,
megafaunal species had similar extinction risks as smaller species, in
stark contrast to modern oceans where the large species are most
affected by human perturbations. Our work represents a first step
towards a better understanding of the marine megafauna that lived in the
geological past. However, more work is required to expand our list of
taxa and their traits so that we can obtain a more complete picture of
their ecology and evolution.
[No figures in the paper, so here's a photo of the Otago Geology Museum and the prep lab, featuring a bunch of artwork by Chris Gaskin, and Gabriel Aguirre-Fernandez for scale].
The 47 vertebrate type specimens held in the University of Otago Geology
Department are catalogued in detail. A short history of the collection
is followed by lists of the type specimens under the Classes
Actinopterygii, Reptilia, Aves and Mammalia. A fish trace-fossil is
included at the end of the Actinopterygii. Where appropriate, the name
changes of the genus or species are given in chronological order. The
specimens are briefly described, locality and geological age information
is provided.
The fossil record of true seals (Family Phocidae) is mostly made up of isolated bones, some of which are type specimens. Previous studies have sought to increase referral of non-overlapping and unrelated fossils to these taxa using the ‘Ecomorphotype Hypothesis’, which stipulates that certain differences in morphology between taxa represent adaptations to differing ecology. On this basis, bulk fossil material could be lumped to a specific ecomorphotype, and then referred to species in that ecomorphotype, even if they are different bones. This qualitative and subjective method has been used often to expand the taxonomy of fossil phocids, but has never been quantitatively tested. We test the proposed ecomorphotypes using morphometric analysis of fossil and extant northern true seal limb bones, specifically principal components analysis and discriminant function analysis. A large amount of morphological overlap between ecomorphotypes, and poor discrimination between them, suggests that the ‘Ecomorphotype Hypothesis’ is not a valid approach. Further, the analysis failed to assign fossils to ecomorphotypes designated in previous studies, with some fossils from the same taxa being designated as different ecomorphotypes. The failure of this approach suggests that all fossils referred using this method should be considered to have unknown taxonomic status. In light of this, and previous findings that phocid limb bones have limited utility as type specimens, we revise the status of named fossil phocid species. We conclude that the majority of named fossil phocid taxa should be considered nomina dubia.
A new long-snouted homodont odontocete fossil from the Lower Miocene of Austria (ca. 22.5–22.0 Ma) is formally described and interpreted based on external skull characteristics supplemented by CT and µCT data. The specimen was found in deposits from the Central Paratethys and includes an incomplete and fragmented cranium and mandible as well as ear bones. It differs markedly from previously identified long-snouted archaic dolphins with single-rooted, homodont dentition, and thus is suggestive of a new taxon, for which the name Romaleodelphis pollerspoecki proposed. The geological context of R. pollerspoecki hints at a neritic habitat in close vicinity to estuarine environments. Romaleodelphis pollerspoecki shares key features with certain members of the so-called ‘Chilcacetus clade’ such as the widening of the premaxillae at the rostrum base and the absence of a deep lateral groove along the rostrum; however, a phylogenetic analysis reconstructs R. pollerspoecki in a polytomy with members of this ‘clade’ and a larger clade including many other taxa. To better understand the phylogenetic relationships of these taxa, comprehensive reexamination of Argryocetus and Macrodelphinus will be necessary. Measurements obtained through µCT-based internal anatomical reconstruction of the bony labyrinth indicate that R. pollerspoecki had the ability to hear specialized high-frequency signals similar to modern narrow-band high-frequency (NBHF) specialists. This work further identifies several extinct platanistoids as hearing within the NBHF spectrum, increasing the understanding of the diversity of ecological adaptations in early-diverging odontocetes and providing more examples of convergent evolution of this hearing type.
Analyses of the cetacean (whale and dolphin) inner ear provide glimpses into the ecology and evolution of extinct and extant groups. The paleoecology of the long-snouted odontocete (toothed whale) group, Parapontoporia, is primarily marine with its depositional context also suggesting freshwater tolerance. As an extinct relative of the exclusively riverine Lipotes vexillifer, Parapontoporia provides insight into a transition from marine to freshwater environments. High-resolution X-ray CT scans (~3 microns or less) of three individual specimens from two species, P. sternbergi and P. pacifica, were acquired. Digital endocasts of the inner ear labyrinths were extracted non-destructively. Nine measurements of the inner ear were compared with an existing dataset covering 125 terrestrial and aquatic artiodactyls. These measurements were then subjected to a principal component analysis to interpret hearing sensitivities among other artiodactyls. Based on our analyses, Parapontoporia was likely to have been able to hear within narrow-band high frequency (NBHF) ranges. This finding indicates another convergence of NBHF-style hearing, or, more intriguingly, suggests that it may be an ancestral characteristic present among the longirostrine dolphins that dominated in the Miocene prior to the evolution of more modern lineages.
We present a technique for the detection of vertebrate skeletons buried
at shallow depths through the use of a ground-penetrating radar (GPR).
The technique is based on the acquisition of high-resolution data by
medium-to-high frequency GPR antennas and the analysis of the radar
profiles by a new forward modelling method that is applied on a set of
representative traces. This approach allows us to obtain synthetic
traces that can be used to build detailed reflectivity diagrams that
plot spikes with a distinct amplitude and polarity for each reflector in
the ground. The method was tested in a controlled experiment performed
at the top of Cerro Los Quesos, one of the most fossiliferous localities
in the Ica Desert of Peru. We acquired GPR data at the location of a
partially buried fossil skeleton of a large whale and analyzed the
reflections associated with the bones using the new technique,
determining the possible signature of vertebrae, ribs, the cranium
(including the rostrum), and mandibles. Our results show that the
technique is effective in the mapping of buried structures, particularly
in the detection of tiny features, even below the classical (Ricker and
Rayleigh) estimates of the vertical resolution of the antenna in civil
engineering and forensic applications.
Sebe et al. Fossils from the Upper Miocene (Pannonian) sands of the Pécsvárad sand pit (Eastern Mecsek Mts., SW Hungary).
The
Mecsek Mountains in SW Hungary represent an uplifted basement block of
the Pannonian Basin. Their Neogene cover includes deposits both from the
Middle Miocene Central Paratethys and from its Late Miocene (Pannonian)
brackish‐water descendant, Lake Pannon. Along the mountain front, the
Pannonian sands of the Pécsvárad sand pit contain a mixed vertebrate
fossil assemblage, which gives insight into terrestrial and aquatic
biota during various time intervals of the Miocene. The fossil‐bearing
sands accumulated between 7.6–6.8 Ma, in a high‐energy littoral setting
of Lake Pannon, as indicated by the mollusc remains. The verte brate
fossil assemblage is relatively diverse compared to the number of finds.
The most abundant group, aquatic mammals, encompasses a minimum of four
odontocete species and a few mysticete taxa, which originally lived in
the Para tethys during the Badenian and the Sarmatian. Rhinocerotid
remains are reworked from sediments aged somewhere between the Karpatian
and earliest Pannonian. The single shark tooth must be Badenian, and
the scombrid fishes probably also date back to that age. Fossils of
other fishes (sparids, latids and acipenserids), giant salamanders,
turtles, crocodilians and cervids might originate from older Miocene
deposits but can be coeval with the host sands as well. Tapirs and
giraffids must have lived on the lakeshores of Lake Pannon, probably
contemporaneously with sand deposition or not much earlier. The erosion,
enrichment and mixing of the fauna is a result of the uplift and
denudation of the Mecsek Mountains during Lake Pannon sedimentation,
caused by neotectonic basin inversion. This example shows that in spite
of the uncertainties in the dating of some fossils, mixed faunas can
provide important data on the evolution history of an area.
Here we report a well-preserved isolated physeteroid tooth of Late
Miocene age from Liessel, the Netherlands. The presence of several
morphological features allows attribution to the macroraptorial
physeteroids. Size and morphology are to some extent comparable to Zygophyseter and almost identical to the primarily tooth-based Tortonian taxon Scaldicetus caretti. However, the genus Scaldicetus was declared unutilizable, which is supported here with an overview of modern classifications of Scaldicetus species and specimens. Despite the restrictions, the type species S. caretti
is still valid, although the name is to be restricted to the type
material. Based on its morphological resemblance, the tooth is
identified as Physeteroidea indet. cf. Scaldicetus caretti.
Eomysticetidae (Chaeomysticeti clade) is an archaic family of baleen whales that originated and diversified during the Oligocene epoch. The fossil record of this family is represented by at least 11 formally described species, including records in Baja California Sur (Mexico), Kaiserswerth (Germany), Kitakyushu (Japan), South Carolina (United States), and South Island (New Zealand). Baja California Sur (BCS) possesses important marine deposits from the upper Oligocene, which document a rich diversity of fossil mysticetes, including members of the family Eomysticetidae. In this work, we report a whale specimen collected in the upper Oligocene San Juan Member, El Cien Formation, from the so-called “Ten Minute locality”, San Hilario, about 100 km NW of La Paz, Baja California Sur. The studied specimen consists of an incomplete skull, in which most of the rostrum and part of the occipital shield are missing but preserving one tympanic bulla in situ. This specimen is identified as belonging to the family Eomysticetidae based on both an elongated skull and nasals and transversely narrow and long intertemporal region with a wide exposure of parietals and frontals. The lack of more diagnostic regions precludes its identification at generic/specific levels. The specimen represents the first description of an eomysticetid for the San Hilario area, increasing our knowledge of the evolution of this group of mysticetes and its record in the Pacific basin from the late Oligocene of BCS.
Modern baleen whales are filter feeders. The Chaeomysticeti is a group of true baleen whales including extinct and all extant baleen whales. The early feeding strategy of the Chaeomysticeti has been discussed but not enough. Considering evolution of feeding strategy of baleen whales, fossils of the Chaeomysticeti are important key to describe how filter feeding in whales began and diversified. To answer such questions, this study examined the relationships of the basihyal-thyrohyal shape and feeding strategy among extinct and extant baleen whales, and hypothesized evolution of the prey types. As the result of analysis, small prey feeders such as balaenids, Caperea marginata, and Eschrichtius robustus share the basihyal-thyrohyal with small articular processes, and a wide and shallow notch between the articular processes. On the other hand, large prey feeders eating fish primarily show very long articular processes and anteriorly oriented lateral portions of the basihyal-thyrohyal, which is a unique condition owned by Balaenoptera edeni among baleen whales. A member of the most basal chaeomysticete: Yamatocetus canaliculatus was plotted close to the cluster of the small prey feeders. This result suggests that the early Chaeomysticeti fed on small prey using the baleen plates for filtering. In the Miocene, the Cetotheriidae and Balaenopteridae started having both large and small prey. Then, a few members of Balaenopteridae such as Balaenoptera musculus and B. edeni were specialized in prey types. In short, prey type of the Chaeomysticeti started from small-sized prey such as small invertebrates then diversified through evolution.
Extant Ganges and Indus river dolphins are endangered species, but their relatives were more diverse in the past. The family Squalodelphinidae is a group of Miocene relatives of Ganges and Indus river dolphins. Our knowledge of squalodelphinids increased slowly in the nineteenth century and has expanded dramatically since the 2010s. Italy, Switzerland, eastern USA, Argentina, and Peru are fossil localities of named and well-preserved squalodelphinid fossils. Squalodelphinids are also known from New Zealand, Germany and Venezuela. However, only two specimens of this family have been reported from the North Pacific, in Washington State, USA, and Japan. Here, a new fossil dolphin (including the skull, right and left periotics and bullae, malleus, incus, hyoid bones and ribs) from the Haze Formation, Ichishi Group, Early Miocene (18.7–18.5 Ma) of Mie Prefecture, Japan is named as the new genus and species Miodelphinus miensis. Phylogenetic analysis places Miodelphinus miensis among squalodelphinids. The periotic of Miodelphinus miensis shows a large, posteriorly widened, ventrally opening, funnel-like articular depression between the posterior and articular processes of the periotic. Miodelphinus miensis contributes to expanding our knowledge of squalodelphinid diversity. Squalodelphinids were distributed widely not only in the Atlantic but also the South and North Pacific by the Early Miocene. This suggests that the family had a chronologically deeper origin such as the beginning of the Early Miocene or older.
Tanaka et al. A new late early to early middle Miocene fossil baleen whale aff. Isanacetus laticephalus specimen from Hokkaido, Japan.
Isanacetus laticephalus is a species of archaic baleen whales, that played a key role to recognize a problematic taxonomic confusion in so-called “cetotheres” for some time. HMG-1475 includes the posterior part of the skull and atlas, which was discovered from Biratori Town, Hokkaido, Japan. Radiolarian fossils suggest that HMG-1475 is late Burdigalian to early Langhian (16.7 to 15.3 Ma) in age. Here, we identify the specimen as aff. Isanacetus laticephalus, because HMG-1475 shares same conditions with Isanacetus laticephalus such having the sagittal crest at the vertex, narrower posterior ends of the premaxillae and nasals, posteriorly gradually converged middle part of the lateral borders of the nasals, anteriorly excavated and rounded border of maxillae at the vertex in dorsal view, and posteriorly well projected paroccipital process in dorsal view. HMG-1475 differs from I. laticephalus by having a distinct temporal crest and a more robust nasal. In Hokkaido, Taikicetus inouei was reported from the Middle Miocene, which is closely related to I. laticephalus. However, HMG-1475 differs from T. inouei by having a sagittal crest between the nasals and supraoccipital, and the temporal crest. HMG-1475 lacks the ear bones and was incompletely preserved. However, it implies that there was an unknown species similar to I. laticephalus around at Hokkaido, Japan (late Early to early Middle Miocene).
Contrasting with their current ranges in the Antarctic and subantarctic zones, the fossil record reveals that phocids (true and elephant seals) inhabited widespread subtropical regions across the Southern Hemisphere in the geologic past. At least four extinct phocid taxa have been described from Miocene and Pliocene fossiliferous levels in Chile and Peru, constituting two of the taxonomically richest phocid assemblages known. Still, some Chilean remains morphologically differ from those recovered from Peru, suggesting an unprecedented phocid diversity. We examined phocid mandibular remains from the Bahía Inglesa Formation in northern Chile. We identified the occurrence of the long-snouted seal Acrophoca longirostris, a morphologically distinguishable and undescribed form of Acrophoca, and Hadrokirus martini, an extinct phocid with a robust feeding morphology, constituting the first record of this taxon outside Peru. We also recognised four other indeterminate phocids with considerable morphological differences from contemporaneous taxa. Moreover, one of these specimens uniquely combines morphological attributes distinct from all known extant and extinct phocids, likely corresponding to a new taxon. These reports significantly increase the taxonomic and morphological diversities of fossil seals from the eastern South Pacific and emphasise the substantial transformations of phocid assemblages over geologic time.
Living pinnipeds play essential roles in marine ecosystems
and display divergent lineage-specific foraging and habitat
preferences. The origin of these ecological strategies remains unclear.
We analyzed original (n = 22) and published (n = 93) stable carbon (δ13C) and oxygen (δ18O) isotope compositions of tooth enamel
from fossil pinnipeds and coeval marine and terrestrial mammals from
the late Early Miocene to early Middle Miocene Temblor Formation, the
early Middle Miocene Round Mountain Silt Formation at Sharktooth Hill
Bonebed, the Middle Miocene of lower levels of the Monterey Formation,
and the late Middle Miocene Santa Margarita Formation from the eastern
North Pacific Ocean; and original (n = 43) δ13C and δ18O
values from the Early Pliocene Yorktown Formation in the western North
Atlantic. As expected for aquatic mammals, pinnipeds and control marine mammals had low δ18O
variability relative to coeval terrestrial mammals, indicating minimal
diagenetic alteration. Among late Early Miocene and Middle Miocene
pinnipeds, Allodesmus had lower δ13C values than coeval taxa indicating offshore foraging, in agreement with predictions based on skeletal morphology. However, Allodesmus from the late Middle Miocene Santa Margarita Formation had overlapping δ13C values with co-occurring cetaceans and pinnipeds suggesting preferential nearshore habitat. The stem otariid Pithanotaria had higher enamel δ13C values than co-occurring pinnipeds, indicating nearshore foraging. The stem odobenid Neotherium had intermediate δ13C values supporting foraging between nearshore and offshore predators. Conversely, the stem odobenid cf. Imagotaria had lower enamel δ18O but comparable δ13C
values to coexisting pinnipeds, suggesting the exploitation of
estuarine resources. At least two ecologically distinct pinniped groups
also occurred in the Yorktown Formation. In this unit, the odobenid Ontocetus and some monachinae phocids were predominantly nearshore foragers, with higher enamel δ13C and δ18O
values than simultaneously occurring marine mammals. The second group
was exclusively composed of phocids, which had comparatively low δ13C and δ18O
values, compatible with northward foraging movements along the western
North Atlantic coast. The paleoecological profiles of fossils bear
strong similarities to the life modes of extant pinniped communities,
implying that extant foraging modes appeared early in pinniped evolution
and that resource partitioning contributed to their community structure
over time.
Adding up to the 36 basilosaurid remains from Bartonian to Priabonian
strata at the open cast mines in the Helmstedt region, Germany that were
published in the past, ten other finds from this region are described
here. Most or all the finds were collected by Rudolf Mundlos in the
second half of the twentieth century. The most significant remains
consist of a fragmentary lumbar (maybe thoracic) vertebra of Pachycetus
sp. with a large part of the neural arch preserved, a radius of a large
archaeocete, and a small posterior thoracic or lumbar vertebral centrum
of a small dorudontine.
Raoellid mammals are small artiodactyls from the Eocene of Asia, hypothesized to be closely related to stem Cetacea. Knowledge of the cranial and dental morphology of Raoellidae comes mostly from one species, Indohyus indirae. Here we describe new material of another raoellid genus, Khirtharia, based on material retrieved from the Kalakot area, Jammu and Kashmir. This new material, comprising an almost complete, lightly deformed cranium and a partial snout with associated partial mandible, greatly adds to our knowledge of raoellid morphology. It highlights the similarity of cranial characters with Indohyus, such as a long snout with raptorial incisors, a thick and narrow supraorbital region, a strong postorbital constriction, a triangular shaped braincase, and a thickened medial wall to the auditory bulla (involucrum). The new specimen is similar to Indohyus cranially but differs dentally in being more bunodont. The presence of these traits in two different raoellid genera suggests they may be present more broadly across Raoellidae. These characters are also observed in early cetaceans, highlighting the need to investigate their phylogenetic impact. Some cranial features support aquatic habits of members of this family.
Raoellidae are small artiodactyls retrieved from the middle Eocene of Asia (ca - 47 Ma) and closely related to stem Cetacea. Morphological observations of their endocranial structures allow for outlining some of the early steps of the evolutionary history of the cetacean brain. The external features of the brain and associated sinuses of Raoellidae are so far only documented by the virtual reconstruction of the endocast based on specimens of the species Indohyus indirae. These specimens are however too deformed to fully access the external morphology, surface area, and volume measurements of the brain. We bring here new elements to the picture of the raoellid brain by an investigation of the internal structures of an exceptionally well-preserved cranium collected from the Kalakot area (Jammu and Kashmir, India) referred to the species Khirtharia inflata. Micro-CT scan investigation and virtual reconstruction of the endocast and associated sinuses of this specimen provide crucial additional data about the morphological diversity within Raoellidae as well as reliable linear, surfaces, and volumes measurements, allowing for quantitative studies. We show that, like I. indirae, the brain of K. inflata exhibits a mosaic of features observed in earliest artiodactyls: a small neocortex with simple folding pattern, widely exposed midbrain, and relatively long cerebellum. But, like Indohyus, the brain of Khirtharia shows unique derived characters also observed in stem cetaceans: narrow elongated olfactory bulbs and peduncles, posterior location of the braincase in the cranium, and complex network of blood vessels around the cerebellum. The volume of the brain relative to body mass of Khirtharia inflata is markedly small when compared to other early artiodactyls. We show here that, cetaceans that nowadays have the second biggest brain after humans, derive from a group of animals that had a lower-than-average expected brain size. This is probably a side effect of the adaptation to aquatic life. Conversely, this very small brain size relative to body mass might be another line of evidence supporting the aquatic habits in raoellids.
The temporalis is an important muscle used in biting and mastication, and whose morphology is strongly influenced by feeding ecology. Although the anatomy of this muscle and its relation to feeding function are well-studied in terrestrial mammals, few studies have examined its variation in whales. Our study focuses on quantifying the area of attachment for the temporalis muscle within the temporal fossa, calculating two metrics of comparison: A temporal fossa index (TFI) which is a size corrected measure of temporalis muscle attachment area, and a corrected temporal fossa index (CTFI), which also corrects for cranial telescoping. We calculated TFI and CTFI scores for 72 species of extant odontocetes as well as 37 species of extinct whale, including archaeocetes and toothed mysticetes. We statistically tested for differences related to diet and prey capture method using ANOVA. We then performed ancestral character state reconstruction (ACSR) for both metrics. We found no significant differences in TFI scores for diet, however both grip-and-tear and snap feeding taxa had significantly larger TFI scores than suction or ram feeding odontocetes. The ACSR found major decreases in TFI at the base of the Neoceti, Odontoceti, and just prior to the evolution of the crown group, relating to the gradual loss of mastication. When we corrected for telescoping, CTFI scores increase within mysticetes and stem odontocetes, before decreasing again within crown odontocetes. This suggests that as telescoping increased, some compensation was needed to account for the reduced intertemporal region. We find evidence of macropredatory behavior for Basilosaurus, Coronodon, Ankylorhiza, Livyatan, and possibly Atocetus. Hyper-longirostrine taxa such as eurhinodelphinids possess unusually low TFI scores, as does the bizarre prognathous porpoise Semirostrum, highlighting their unique feeding styles. Our study reveals a complex interplay between the reduction in mastication, increase in cranial telescoping, and prey capture specialization. It also introduces a useful and simple metric which can be used to infer feeding ecology in fossil whales.
Special Volume of the Bulletin of the Mizunami Fossil Museum
There is an entire special volume of the Bulletin of the Mizunami Fossil Museum (a long-running, but intermittently published journal) dedicated to the discovery, context, exhibition, and initial study of a beautifully preserved Paleoparadoxia skeleton from Mizunami City in Gifu Prefecture (island of Honshu, a little over 200 km west of Tokyo). The articles, aside from the preface, have abstracts in English, but the main text is all in Japanese; a few figures are included above.
Ando. Study of the "Paleoparadoxiid Mizunami-Kamado specimen" (Preface).
A well-preserved intact skeleton of paleoparadoxiid was
excavated from the river bed of the Toki River in Shimogiri, Kamado-cho,
Mizunami City, Gifu, central Japan in June 10th, 2022. We call this
specimen “Paleoparadoxiid Mizunami-Kamado specimen” (MFM 18130). It is
from the Lower Miocene Shukunohora Formation of Mizunami Group (ca. 16.5
Ma). The skeleton is nearly complete, including the skull, mandible,
vertebrae, ribs, hindlimbs, and all sternal bones. The pa-per records
and figures the excavation and preparation process (June, 2022 to
August, 2023) of “Paleoparadoxiid Mizunami-Kamado specimen”.
Paleoparadoxiid fossils were discovered in the Shukunohora
Formation of the Mizunami Group exposed in the Toki River bed in
Shimogiri-ku, Kamado-cho, Mizunami City, in 2022. In order to clarify
the age of the Shukunohora Formation in this locality, we determined
stron-tium isotopic ages using shell fossil samples that were cooccurred
with paleoparadoxiid fossils. The strontium isotopic ages suggest that
the depositional age of the paleoparadoxiid-bearing horizon is ca. 16.5
Ma.
This paper presents the results of magnetic measurements on
the sandstone block containing the “Paleoparadoxiid Mizunami-Kamado
specimen” from the Shukunohora Formation of the Miocene Mizunami Group
in central Japan. Stepwise demagnetization suggests the presence of both
mag-netite and greigite, with the latter being probably authigenic
minerals formed during early diagen-esis. The anisotropy of magnetic
susceptibility (AMS) analysis shows a highly variable magnetic fabric
with no preferred orientation, suggesting the absence of a consistent
grain alignment in the sandstones. This likely reflects disturbances by
storms or bioturbation. The sandstone block-mean remanent magnetization
direction may be attributed to chemical remanent magnetization (CRM)
carried by greigite and, therefore, may not represent the paleofield
direction at the time of deposi-tion. However, assuming CRM acquisition
during early diagenesis shortly after deposition, the deposition was
during a reverse polarity period around 16.5 Ma, potentially either
Chron C5Cr (17.154–16.637 Ma), C5Cn.2r (16.532–16.434 Ma), or C5Cn.1r
(16.351–16.261 Ma). The devia-tion of the block-mean remanent
magnetization direction from the geocentric axial dipole field suggests a
clockwise tectonic rotation (28 ± 20°) for the crust beneath the study
area relative to the Asian continent. This rotation coincides with the
clockwise rotation of Southwest Japan during the major opening of the
Japan Sea, suggesting the “Paleoparadoxiid Mizunami-Kamado specimen”
lived amidst this tectonic event.
“Paleoparadoxiid Mizunami-Kamado specimen” is a
well-preserved whole skeleton of paleoparadoxiid that was excavated from
Kamado-cho, Mizunami City Gifu prefecture in June 2022. Each bone is
preserved very well, and the bone and other fossils have plenty of
information about the situation before they became fossilized.
Therefore, this study records the positions of each body part of this
specimen and other fossils preserved together with the specimen to
discuss the taphonomy and the growth of bones of the specimen. A total
of 119 bones were collected, including the skull, mandible, vertebrae,
ribs, hindlimbs, and sternal bones of the specimen, but it lacks the
forelimbs. The growth of these bones was almost stopped, which suggests
the specimen is a mature individual. After comparison with other
specimens of paleoparadoxiid, the specimen in this study turned out to
be one of the most mature specimens of Paleoparadoxiidae. Fossil remains
of molluscs, barnacles, decapods, sharks and plants were col-lected
from around the specimen. Some barnacles and molluscs attached to some
bones. The infor-mation of this specimen and other fossils suggest that
the carcass drifted on the sea before going down to the sea bottom and
took much time to deposit.
A nearly complete
paleoparadoxiid skeleton, i.e., MFM 18130, from the Lower to Middle
Miocene Shukunohora Formation, Kamado Town, Mizunami City, Gifu
Prefecture, Japan, is identified based on the cranial, mandibular and
postcranial characters. MFM 18130 has such unique characters as
pres-ence of the postzygomatic foramen on the skull, collumnar cusps on
the molars, paired sterna, strongly twisted tibia and astragalus, and
therefore, belongs in the family Paleoparadoxiidae of the Desmostylia.
In addition, MFM 18130 has some derived characters that are possessed in
the genera Archaeopara-doxia and Neoparadoxia such
as dorsally high suprraoccipital process and shortened zygomatic
pro-cess. On the contrally, the conditions of these characters are
opposite in the monospecific Paleopara-doxia. In addition, the presence of the hypoconulid on M/3 is different from that of the monospecific Archaeoparadoxia, and the body size is much smaller than any species of Neoparadoxia. Accordingly, MFM 18130 is provisionally identified as Paleoparadoxiidae genus and specis undetermined.
A paleoparadoxiid specimen from Kamado, Mizunami exhibits
numerous bone fragment-like fossils rang-ing in diameter from a few
millimeters to about 3 centimeters. These fossils are associated with
various morphological features, such as rod-like arrangements depending
on the preservation state, surface features rich in irregularities and
nutrient foramina, and histological characteristics including a
peripheral compact layer composed of calcified cartilage and a spongy
interior composed of bone tissue. Based on these features, we
preliminarily identify the segmented structure as mineralized tissue
developed within the costal cartilage. Most of the isolated bone
fragment-like fossils probably have belonged to mineralized costal
cartilage judg-ing from the morphological and histological similarity to
the elements of the rod-like aggregate. The struc-ture and
mineralization process of rib cartilage in desmostylians, including the
family Paleoparadoxiidae, remain unclear, and this collection of rib
cartilage specimens is expected to serve as a novel source of
infor-mation for clarifying the physiology, ecology, and phylogeny of
desmostylians. Future research should fo-cus on further elucidating the
details of rib cartilage fossils in the Mizunami-Kamado specimen and
explor-ing the relationships between the pattern of rib cartilage
mineralization and ecology or physiology by com-prehensively
investigating the morphological features of rib cartilage in various
mammalian lineages. This approach is anticipated to provide new
biological insights into desmostylians.
Formation processes of calcite concretions co-occurred with the “Paleoparadoxiid Mizunami-Kamado specimen” and calcite fillings formed in bones were studied and discussed based on ge-ological observations and geochemical analyses. The un-deformed fecal pellet-like structures in concretion show that concretions formed before the compaction of sediments. The low δ13C values (approximately −10‰) of concretions and calcite fillings in bones and similar δ18O values to each other suggest that they were formed from organic carbon at almost the same time during early diagenesis. The calcite fillings in bones protect the skeleton from deformation due to the compac-tion of sediments.
Ando. Molluscan fossils from the locality of the “Paleoparadoxiid Mizunami-Kamado specimen” of the Shukunohora Formation, Mizunami Group in Mizunami City, central Japan
Molluscan fossils including Crenomytilus grayanus (Dunker) and Chlamys itoigawae Masuda
were collected from the locality of the “Paleoparadoxiid
Mizunami-Kamado specimen” in Kamado-cho, Mizunami City, Gifu Prefecture,
central Japan. The specimen of Mytilus coruscus Gould reported by Itoigawa et al. (1974, 1981, 1982) and Taguchi (2002) are reexamined, and these specimens are referable to C. grayanus by
having fine radial striae on the shell exterior, fine crenulations on
the interior shell margin and a non-vacuolated psedonymph.
Many specimens of a
small echinoid (sand dollar) species were collected from the excavation
site of paleoparadoxiid in Kamado, Mizunami, central Japan. These are
safely assigned to Kewia minoensis because
of their morphology and their smallness. Occurrence of these echinoid
enables to reconstruct the paleoenvironment as a purely marine
environment without influence of brackish water from nearby.
Total 124
elasmobranch teeth were co-occurred with “Paleoparadoxiid
Mizunami-Kamado speci-men”. Ninety-seven specimens were identified with Galeocerdo aduncus, and other teeth were also identified with genera Carcharhinus and Squatina, except for one batoid tooth. Many bite marks were recognized on the left femur, middle phalanx of left hindlimb and left 14th to 15th ribs. The present paleoparadoxiid seemed to be preyed upon those sharks.
Twenty-seven species of fossil Ostracoda, 17 species of
planktonic Foraminifera, and 18 species of benthic Foraminifera were
found in sandstones around the paleoparadoxiid-bearing horizon of the
Miocene Shukunohora Formation, Mizunami Group, central Japan. Based on
the results of the microfossil analysis, the depositional age was
assigned to the lower part of planktonic foraminif-eral zone N8
(~17.0–16.0 Ma) during the Mid-Miocene Climatic Optimum. The
paleoenvironment is thought to have been sandy sea bottoms in upper
sublittoral zone (~20–50 m) near seaweed or seagrass beds under the
influence of strong warm currents.
The pollen
assemblage from the sediment sample containing the whole skeleton of a
paleopara-doxiid (“Mizunami-Kamado specimen”), is mainly composed of Pinus, Dacrydium, Quercus (evergreen type) and others. This assemblage includes warm elements such as Keteleeria, Pseu-dolarix, Quercus (evergreen type), Dacrydium, Liquidambar, and Reevesia,
many of them are now extinct in Japan. The assemblage is comparable
with the pollen assemblages previously documented from the Shukunohora
Formation, Mizunami Group, Gifu Prefecture, Japan. These assemblages
correspond to the NP-2B pollen zone, indicative of the Middle Miocene
climatic optimum. The paleovegetation is inferred to be evergreen
conifer forests characterized by Pinus and Dacrydium with evergreen broad-leaved trees such as evergreen Quercus and
deciduous broad-leaved trees. The paleoclimatic conditions are
estimated to be about 21°C for the annual mean temperature with an
annual precipitation of 1,500–1,800 mm. Similar pollen assemblages found
in sediments containing Paleoparadoxia in Niigata and Okayama prefectures also repre-sent the NP-2B pollen zone and include mangrove plant pollen fossils.
Laser scanning and X-ray CT scanning were performed on
“Paleoparadoxiid Mizunami-Kamado specimen” to determine the condition of
the vertebrae, right hindlimb, and skull buried in rock, which are
extremely difficult to extract due to their state of preservation, to
observe the interior of the skull, and to create 3D digital
reconstructions of the skeleton. In addition, not only bone elements but
also changes in the specimen due to the preparation process were also
digitized. The digitization of the bone elements of the specimen made it
possible to easily create whole-body skeletal reconstruction models,
which will be a precious re-source for research and educational
purposes.
Oda et al. Procedures of paleoillustration of the “Paleoparadoxiid Mizunami-Kamado specimen”
A paleontological restoration of the “Paleoparadoxiid
Mizunami-Kamado specimen” which was ex-cavated from the Shukunohora
Formation of the Mizunami Group in Kamado-cho, Mizunami City, Gifu,
Japan, was created as a 2D illustration. The form and ecology of when
the specimen was preyed by sharks, were reconstructed based on skeleton
of MFM18130, its burial position. The illustration was revised by the
Kitagawa and Kohno’s scientific comments before its completion.